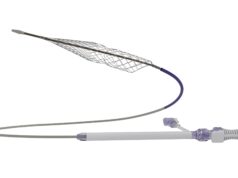
Surmodics has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
Surmodics has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
The Pounce XL thrombectomy system is indicated for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature in vessels 5.5–10mm in diameter, making it suitable for iliac, femoral, and other arteries within this range. The Pounce XL thrombectomy system dramatically increases the size range of the Pounce platform, which also includes the thrombectomy system, indicated for 3.5–6mm peripheral arteries, and the Pounce Low Profile (LP) thrombectomy system, indicated for 2–4mm peripheral arteries. The Pounce thrombectomy system and Pounce LP thrombectomy system were introduced in 2021 and 2024, respectively.
“Securing FDA clearance for Pounce XL is a major step forward in Surmodics’ pursuit of a complete mechanical thrombectomy solution for all peripheral arteries, notably critically ischemic lower extremity vessels,” said Gary Maharaj, president and chief executive officer of Surmodics. “The Pounce thrombectomy platform has already demonstrated its performance as a rapid, efficient solution for the removal of both acute and chronic thrombi and emboli in peripheral arteries without the use of thrombolytics. The addition of the Pounce XL thrombectomy system to our Pounce thrombectomy platform demonstrates our commitment to setting the pace and direction of innovation in this critical space.”
Maharaj added: “Critically ischaemic peripheral arteries often have older, organised clots that resist catheter-directed thrombolysis and aspiration thrombectomy. The Pounce thrombectomy platform allows physicians to rapidly restore blood flow regardless of clot morphology, which has the potential to reduce the need for follow-up procedures and additional thrombolytic therapy requiring intensive-care-unit admission.”
Surmodics expects to initiate limited market release for the Pounce XL thrombectomy system in the first half of 2025, with commercialisation planned following the completion of the limited market release.