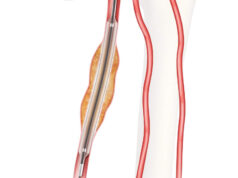
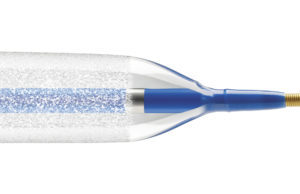
 Spectranetics has announced receipt of US Food and Drug Administration (FDA) premarket approval of the Stellarex drug-coated balloon (DCB), designed to restore and maintain blood flow to the superficial femoral and popliteal arteries in patients with peripheral artery disease (PAD).
Spectranetics has announced receipt of US Food and Drug Administration (FDA) premarket approval of the Stellarex drug-coated balloon (DCB), designed to restore and maintain blood flow to the superficial femoral and popliteal arteries in patients with peripheral artery disease (PAD).
“The Stellarex DCB has shown safety and efficacy in a cohort of patients which included a higher preponderance of diabetics and core-lab defined severely calcified lesions in the US IDE trial. These results, in combination with the cohort of patients studied in the European randomised controlled trial, demonstrate the Stellarex DCB is safe and effective in diverse patient populations when addressing symptomatic femoropopliteal disease,” stated Prakash Krishnan, ILLUMENATE pivotal trial’s co-principal investigator, Mount Sinai, New York, USA.
Sean Lyden, ILLUMENATE’s co-principal investigator, Cleveland Clinic, Cleveland, USA, added, “The ILLUMENATE pivotal trial treated a challenging patient population with a low paclitaxel drug dose. When considering options, I think most clinicians would prefer to use a lower drug dose if they can also achieve great patency results.”
Throughout the ILLUMENATE trial series, Stellarex DCB’s EnduraCoat technology demonstrates both safety and efficacy with consistently high patency rates and low clinically-driven target lesion revascularisation rates in a range of lesion complexities and patient co-morbidities. The device offers both hybrid paclitaxel crystallinity and a durable excipient to provide efficient drug transfer, effective drug residency with high coating durability and minimal particulate loss.
“The Stellarex DCB delivers top-tier clinical results for common to complex patients. As the only commercially-available DCB with two reported randomised controlled trials, Stellarex has demonstrated durability with consistently high patency rates in a wide range of patients,” said Scott Drake, president and chief executive officer of Spectranetics. “Our customers refer to Stellarex as a no-compromise solution. This next generation DCB provides proven treatment backed by robust clinical evidence.”
“Spectranetics is a provider of proven solutions to manage the challenges of crossing lesions, preparing vessels, and treating even the most complex coronary and peripheral lesion morphologies. We are always focused on better patient care, and with today’s FDA approval, the Stellarex DCB becomes a formidable competitor in the drug-coated balloon market, and a proven solution for those suffering with PAD,” said Scott Hutton, senior vice president and general manager at Spectranetics.