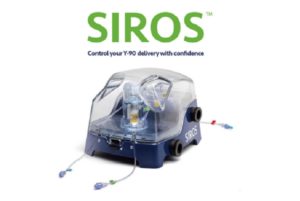
 Sirtex Medical has today announced that it has received certification according to European medical device regulation (MDR) 2017/745 for SIR-Spheres Y90 resin microspheres and its delivery systems, paving the way for the European launch of the SIROS delivery system.
Sirtex Medical has today announced that it has received certification according to European medical device regulation (MDR) 2017/745 for SIR-Spheres Y90 resin microspheres and its delivery systems, paving the way for the European launch of the SIROS delivery system.
The MDR places greater emphasis on patient safety measures, risk management, post-market surveillance, and comprehensive data collection for medical devices seeking European market access.
“Patient health and safety are always at the forefront of our work. That is why we are particularly proud to receive EU MDR certification for SIR-Spheres Y90 resin microspheres and its delivery systems, including SIROS. This achievement underscores our commitment to delivering innovative products responsibly and ethically,” said Matt Schmidt, chief executive officer of Sirtex Medical. “With the expansion of SIROS outside of the USA, interventional radiologists in Europe now have an additional option to support patients battling metastatic colorectal cancer (mCRC) and unresectable hepatocellular carcinoma (HCC). This milestone reflects the outstanding efforts of our team, the rapid growth of our business, and the groundbreaking interventional oncology solutions we have developed.”
The SIROS delivery system offers an intuitive, visual, and adaptable solution for physicians to administer SIR-Spheres to liver cancer patients. Its easy-to-use design along with controlled administration provides physicians confidence in the Y90 delivery, states a recent press release published by the company.
“As an interventional radiologist, I am excited about the future use of the SIROS Delivery System,” shared Thomas Helmberger (München Klinik Bogenhausen, Munich, Germany). “This device has an intuitive design and a precise control that will facilitate the administration of SIR-Spheres Y90 resin microspheres, and I am looking forward to incorporating it into my practice.”