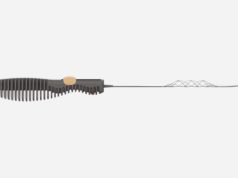
Rapid Medical has announced its Drivewire novel guidewire with a steerable distal top, has received US Food and Drug Administration (FDA) clearance. According to a company press release, Drivewire is the first neurovascular guidewire with a controllable distal end that can change course and shape on-demand for more precise navigation.
Rapid Medical states that, with Drivewire, physicians control the direction and shape of the guidewire’s tip while it is inside the vessel, precisely navigating the neuro and peripheral vasculature. Rapid Medical add that this intravascular steering provides access to difficult anatomical locations, increasing the physician’s ability to treat vascular diseases with less invasive interventional approaches.
The press release states that guidewires are key components for treating intravascular diseases such as ischaemic and haemorrhagic strokes. Rapid Medical claims that currently physicians do not have direct control over the guidewire tip and often remove the guidewire several times to reshape it before reaching the desired location.
Erez Nossek, (NYU Langone, New York, USA), commented: “The development of Drivewire has been an exciting collaboration. I can now easily navigate through complex anatomies by varying the shape of the wire tip inside the vessel, something that cannot be performed with the current neurovascular guidewires.
“I expect this first-of-its-kind technology to benefit patients with challenging anatomies and increase the interventional treatment options available to them.”
Outside of the US the device is named Columbus.