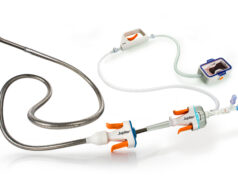
Boston Scientific announced positive results for the EkoSonic endovascular system (EKOS system) during a late-breaking clinical trial presentation at Vascular Interventional Advances (VIVA) 2021 (5–7 October, Las Vegas, USA). Data from the KNOCOUT PE registry—established to measure institutional adoption of a lower dose and lower-duration thrombolysis protocol for the EKOS system—confirmed the safety and efficacy of the EKOS system for the treatment of patients with intermediate-high and high-risk pulmonary embolism (PE).
“PE remains a life-threatening and complex disease, but these results provide an opportunity to advance patient care by showcasing evidence that proves a lower drug dose and shorter infusion duration of a thrombolytic agent may result in enhanced safety and efficacy,” said Keith M Sterling (Inova Alexandria Hospital, Alexandria, USA), study principal investigator. “The findings in this registry analysis are very reassuring to physicians making critical evidence-based decisions for their patients in what are oftentimes emergent treatment situations.”
The international registry of 489 patients across 83 centres included data from patients treated with the EKOS system who were provided a lower drug dose and shorter infusion duration of a thrombolytic agent than administered in previous studies, reflecting contemporary clinical practice. In the data, there were no intracerebral haemorrhagic (ICH) events, or brain bleeding events, with a low major bleeding rate of 2.5%, compared to the rate previously observed with systemic thrombolysis treatment. Results also demonstrated a 23% post-procedure reduction in the main indicator of heart strain from PE, measured as right ventricular to left ventricular diameter ratio (RV/LV).
“As the largest prospective body of evidence in the interventional PE space to date, the KNOCOUT PE registry provides an accurate modern representation of patients with PE treated with the EKOS system every day,” said Michael R Jaff, chief medical officer and vice president clinical affairs, technology and innovation, Peripheral Interventions, Boston Scientific. “The strong safety and efficacy findings exhibited in this registry add to the existing clinical evidence supporting the EKOS system as a treatment option that physicians can trust, as it is already the most studied interventional device in the PE space.”
The ultrasound technology used by the EKOS system accelerates thrombolysis—the breakdown of the clot—minimising the time it takes to treat a patient and lowering the necessary thrombolytic dose, which can result in optimised outcomes and a lower risk of bleeding.