
Following his statement titled ‘Paclitaxel meta-analyses in the lower limbs: Missing the trees for the forest’ published in the June issue of The Journal of Vascular and Interventional Radiology (JVIR), Konstantinos Katsanos (University Hospital Patras, Rio, Greece) imparts his final word on the paclitaxel controversy, surveying the key meta-analyses data that moved the needle.
More than five years ago we documented an increased long-term risk of all-cause death with the use of paclitaxel-coated devices in the femoropopliteal artery that sparked an intense scientific debate and flurry of research articles pursing to elucidate the actual risk of death associated with such devices in the peripheral arteries.1 Of note, our findings were soon corroborated by an individual patient data (IPD) meta-analysis of US Food and Drug Administration (FDA)- regulated paclitaxel devices.2 However, several follow-up observational studies have failed to replicate the survival detriment in real-world cohorts and the latest version of a similar IPD meta-analysis with more complete follow-up has found no significant mortality risk.3 Consequently, several regulatory agencies have recently exonerated paclitaxel from the death stigma in the lower limbs.
Certainly, this remains one of the most puzzling scientific questions and one needs to carefully review the design and quality of enrolled studies, the dosage and pharmacokinetics of the devices tested, the patient case mix differences, and of course the rigour of the statistics methods employed in the analyses. For starters, cause-and-effect questions (i.e. does paclitaxel in the legs increase risk of patient death?) are best answered by appropriately designed randomised controlled trials (RCTs) alone that by design suffer from reduced confounding and other bias compared to observational studies. To build on this, a recently updated study-level summary of 27 studies with 5,502 patients produced a significant risk ratio of 1.28 (95% confidence interval [CI], 1.04–1.58; random effects model) at two years, contrary to the reassuring patient-level results of Parikh et al from 10 RCTs with 2,666 patients at 4.9 years (Hazard ratio [HR]: 1.14, 95% CI (0.93–1.40), frailty Cox model).3,4
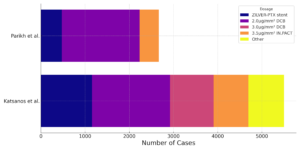
From a critical perspective, the latter meta-analysis of industry-sponsored analyses are missing a significant number of studies from our original 2018 meta-analysis with selective inclusion of mostly low-dose trials.3 Figure shows a ChatGPT-powered visualisation of a stacked horizontal bar chart of sample size contrasting Parikh et al’s latest paper with the totality of RCT evidence presented in the Katsanos et al study. The chart illustrates proportionally the sample size of devices with different design/dosage that are distinguished by colour, including ZILVER-PTX stent, 2.0μg/mm² DCB, 3.0μg/mm² DCB, 3.5μg/mm² IN.PACT, and other (e.g. SWEDEPAD study). Apparently, the latest IPD analysis suffers from selection bias being dominated by the low-dose devices and missing a significant amount of evidence from the rest of the devices. Therefore, the original paclitaxel mortality signal has been obviously diluted, but most likely not invalidated.4
So, how can we consolidate our understanding so far of the relationship between paclitaxel in the lower limbs and potential risk of death, and what should be the next steps? Certainly, the subgroup of low-dose paclitaxel-coated balloons has appeared to be safe since our original meta-analysis, but the IPD data silo built by the industry (with the exception of Cook Medical) along with the complexity of paclitaxel chemistry, pharmacology and tissue bioavailability still precludes any definitive conclusions. In addition, upon observation of diverse treatment effects between trials and time periods, one also needs to consider patient case mix differences and changes in medical science and practice over time. Therefore, an informed patient and doctor’s decision remains the wisest choice as advocated by our teachers a long time ago.
References:
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc 2018; 7:e011245.
- Rocha-Singh KJ, Duval S, Jaff MR, et al. Mortality and Paclitaxel-Coated Devices: An Individual Patient Data Meta-Analysis. Circulation 2020; 141:1859-1869.
- Parikh SA, Schneider PA, Mullin CM, et al. Mortality in randomised controlled trials using paclitaxel-coated devices for femoropopliteal interventional procedures: an updated patient-level meta-analysis. The Lancet 2023; 402:1848-1856.
- Katsanos K. Journal of Vascular & Interv Radiol Published:June 04, In press, 2024 DOI:https://doi. org/10.1016/j.jvir.2024.05.018.
Konstantinos Katsanos is a consultant vascular and interventional radiologist, specialising in endovascular, spine and oncology at the University Hosptial Patras, Rion, Greece.










