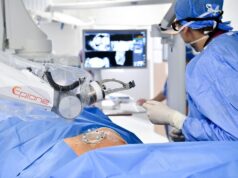
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Mendaera’s Focalist, a handheld robotic system designed to improve the precision of ultrasound-guided needle placement.
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Mendaera’s Focalist, a handheld robotic system designed to improve the precision of ultrasound-guided needle placement.
According to the company in a recent press release, the system combines real-time imaging with a handheld robotic control to assist clinicians in performing a wide range of minimally invasive procedures and settings.
Its features include robotic needle positioning, touchscreen targeting, and continuous needle depth-tracking.
With the FDA clearance now secured, the company is planning a limited launch of the system in select medical institutions throughout the year, initially targeting the field of urology.
The system is said to support procedures such as percutaneous nephrolithotomy (PCNL), which requires precise kidney access.
The full commercialisation of the system is expected to take place in 2026.
Focalist received clearance for use in adult and paediatric patients for guiding the placement of interventional devices relative to the ultrasound transducer to aid in diagnostic or therapeutic procedures.
Mendaera chief executive officer and co-founder Josh DeFonzo said: “Precise placement of needles to perform a wide range of procedures—organ access, biopsies, vascular access, or therapy delivery, as examples—is a very challenging, but foundational technique that underpins most patient care journeys.
“Our mission is to ensure that these procedures are delivered safely and efficiently across the healthcare system by enabling more providers with the confidence needed to perform these techniques. Robotics have been shown to increase provider capabilities in complex procedures, and we intend to demonstrate that the same can be true in everyday care.”