The use of intra-arterial delivery for the treatment of malignant brain tumours is safe and provides clinical benefits in terms of survival when used with the appropriate therapeutic agents, according to a small phase II study presented at the virtual European Conference on Interventional Oncology (ECIO; 24 February, online). Delivering these results, Gérald Gahide (Sherbrooke University Hospital, Sherbrooke, Quebec, Canada) told delegates that while the present study demonstrated the technical feasibility and safety of this application, “there is a dire need for designing multicentre, prospective, phase III studies to properly compare intra-arterial and intravenous treatments”.
Beginning his talk with reference to the “important” primary malignant cerebral nervous system tumours, Gahide explained that both glioblastomas and lymphomas are quite rare (with an incidence of 8.85 out of 100,000 and seven out of 1,000,000, respectively, of the adult population in the USA). Standard treatment for the former tumour is cytoreductive surgery, “meaning you remove as much of the tumour as possible without harming the patient—it is a debulking strategy, used in conjunction with complementary external beam radiation and chemotherapy (mostly with temozolomide)”. The median survival time is 14.6 months. Gahide told delegates: “Unfortunately, it is impossible to remove all of the tumour because it is a very infiltrated disease and relapse is the norm, and five-year survival is less than 10%”.
Commenting on the treatment of cerebral nervous system lymphoma, he said: “The only point that people agree on is that there should be high IV [intravenous] dose of methotrexate (3g/m2) during the induction, but as of today, there is no consensus regarding what other drugs to use for induction or consolidation.”
One of the main reasons explaining the poor prognosis and responses to treatment is the presence of the blood-brain barrier that presents “significant obstacles” to drug delivery, Gahide explained. The blood-brain barrier selectively transports substances into the brain, maintaining the right concentration of essential compounds and protecting the central nervous system from harmful substances. Typically, small (less than 180Da) and lipophilic molecules (oxygen, carbon dioxide, ethanol) diffuse freely and enter a normal blood-brain barrier. Larger hydrophilic molecules need an active transcellular transport to gain access to the central nervous system. According to reports in the literature, 98% of small drugs do not cross the blood-brain barrier.
“Because of these restrictive entities, the role of potentially active chemotherapeutic agents remains marginal in the treatment of malignant astrocytomas,” Gahide said.
Giving an example, he discussed the use of temozolomide in the treatment of primary brain tumours. The intratumoural concentration of the chemotherapeutic agent reaches “only” 20–30% of the serum concentration, he reported, and less than 10% of cerebral metastases reach 10% of the drug concentration measured in the other organs. “So how can we improve that?” he asked.
Intra-arterial drug delivery technique is “quite straightforward”
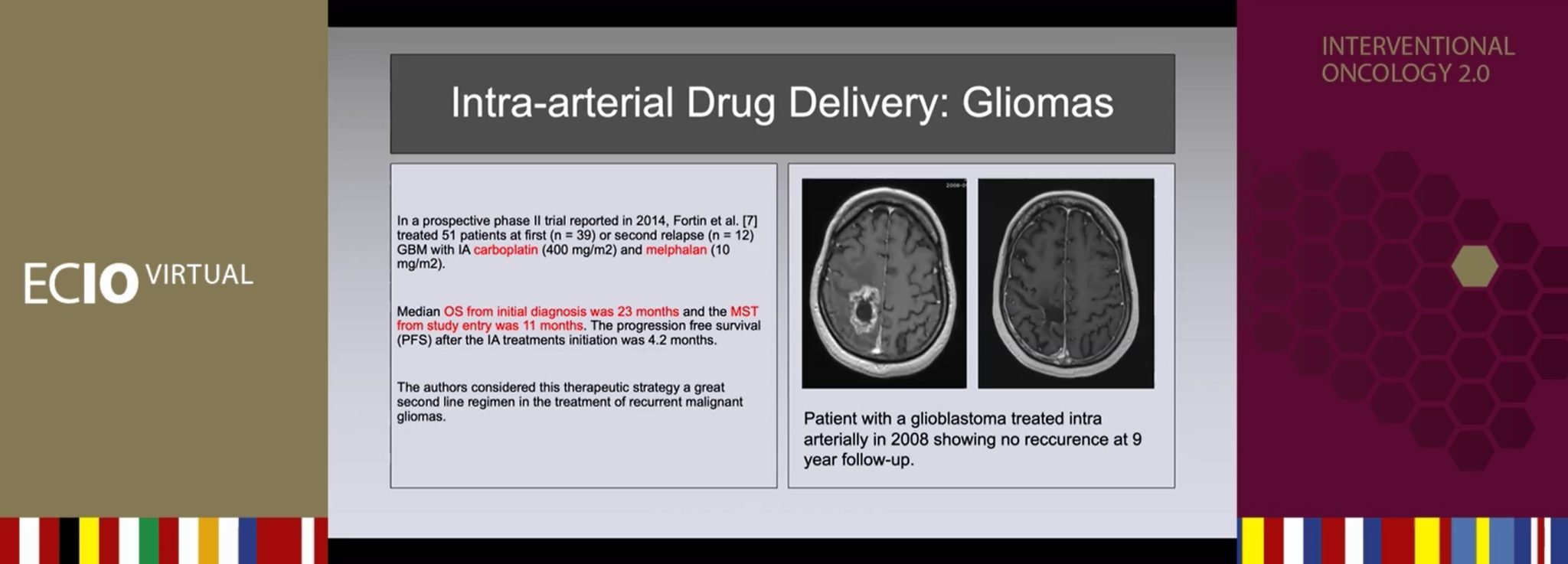
According to Gahide, the technique used for intra-arterial drug delivery is “quite straightforward”. His team take a femoral approach: “It is a conscious sedation,” he explained, “and we place a catheter in to the carotid or the vertebral artery, depending on the location of the tumour.” Angiography is used to confirm the position of the catheter and the integrity of the cerebral arteries. Patients must have a measurable disease on initial contrast-enhanced MRI scans, and a Kamofsky performance score >50. Chemotherapies are infused with an automated injector: carboplatin, 400mg/m2, 20cc/min; melphalan, 10mg/m2, 20cc/min. Treatment takes place every four weeks (representing one cycle) for up to 12 cycles after a systematic monthly MRI scan.
A history of intra-arterial delivery
In 1950, Calvin Klopp (George Washington University, Washington, DC, USA) reported the first intra-arterial delivery of chemotherapy for nine head and neck tumours, including one glioblastoma.
The rationality behind this approach is that you can increase the local plasma drug concentration via a first pass effect—Gahide noted that it has been demonstrated that you can increase the intratumoural concentration by up to 90% with this approach compared with systemic (IV) delivery.
In addition, despite Gahide recounting how early studies showed severe brain toxicity (including reports of encephalitis and brain necrosis) triggered by chemotherapeutic agents, he reassured the ECIO audience that “we now have a panel of drugs that have demonstrated their efficacy and their innocuity when used intra-arterially for brain tumours treatment.”
Osmotic blood-brain barrier disruption technique can increase intratumoural drug concentration to more than 300 times that of IV
Another way of circumventing the blood-brain barrier is to open it, Gahide said, introducing the concept of osmotic blood-brain barrier disruption (OBBBD). “In order to improve on the delivery obtained with IA [intra-arterial] infusion, the idea of osmotic manipulations for transiently opening the blood-brain barrier has been explored,” he said. “IA infusion of a hyperosmolar agent causes a rapid diffusion of fluid out of the cells, shrinkage of the endothelial cells, and widening of the tight-junctions. Of the many hypertonic solutions with potential to disrupt the blood-brain barrier, mannitol is the typical choice in both preclinical and clinical studies. This increase in blood-brain barrier permeabilisation is transient in the ipsilateral hemisphere and lasts from 30 minutes to two hours.”
Preclinical studies consistently showed higher intratumoural concentrations with OBBBD delivery compared to intra-arterial infusions or systemic administration. It has been reported in the literature that IA infusions with blood-brain barrier disruption increased the intratumoural concentration of carboplatin 18-fold compared with IA administration alone, and 320-fold compared with IV.
“We use it mostly for lymphomas and in young patients with recurrent glioblastomas,” Gahide commented. Patients must have a Kamofsky performance score >50, and the absence of a significant effect on MRI scan. The procedure is performed under general anaesthesia, unlike intra-arterial delivery alone, and Gahide describes how he and his team take a transfemoral approach to the main artery perfusing the tumour. The catheter is placed at the level of C2–C3 for the carotid circulation, and at the level of C3–C4 for the vertebral circulation. The interventionalists obtain angiographic confirmation of the position of the catheter and integrity of the cerebral arteries, and infuse mannitol at high flow for 30 seconds. A second angiogram is taken to ascertain vessel integrity, before chemotherapy is infused at a constant rate with an automated injector to prevent streaming. Like more traditional IA delivery, patients are treated every four weeks for 12 cycles.