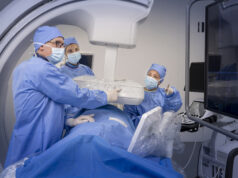
Hansen has announced the completion of the world’s first clinical procedure with the Magellan 10F robotic catheter. Interventional radiologist Gerard Goh performed the procedure at The Alfred Hospital in Melbourne, Australia. He used the 10F catheter during a robot-assisted placement of an inferior vena cava filter.
The procedure was supported in partnership with Medtel Pty, Hansen’s exclusive distribution partner for the Magellan robotic system in Australia and New Zealand.
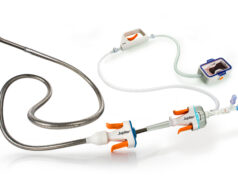
The Magellan 10F robotic catheter is the latest addition to the family of robotic catheters used with the Magellan robotic system. The 10F catheter allows for independent, robotic control of two telescoping catheters (an outer guide and an inner leader catheter). The guide catheter has a 10F outer diameter, and features the largest inner lumen (7F) in the Magellan catheter family, which enables delivery of therapeutic devices through the robotic catheter in a broader range of endovascular procedures. The Magellan 10F robotic catheter received CE mark approval in April 2015.
“The Magellan 10F robotic catheter will enable us to expand our use of intravascular robotics in minimally-invasive, endovascular procedures,” said Goh. “The larger, 7F inner lumen accommodates many more therapeutic devices, including stents, balloons, and in this case, an IVC filter. The device tracked well through the catheter, and we were able to benefit from the robotic precision and stability that we have experienced during previous therapy delivery with the Magellan 6F and 9F catheters.”
The Magellan system’s remote workstation allows physicians to control robotic catheters and guide wires while seated away from the radiation field, which has been shown to reduce radiation exposure for the physician by as much as 95% in complex endovascular procedures.