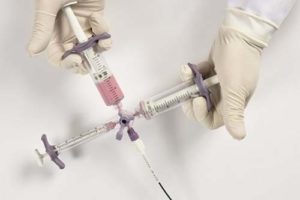
Guerbet has announced obtaining the CE mark for its conventional transarterial chemoembolization (cTACE) mixing and injection system, Vectorio.
Designed in collaboration with interventional radiologists worldwide, Vectorio is a unique set of Lipiodol resistant medical devices including syringes, patented stopcock and sampling devices. Vectorio is dedicated for mixing and delivering Lipiodol ultra fluid and anticancer drugs during cTACE procedure in adults with known, intermediate-stage hepatocellular carcinoma (HCC).
Advantages
This medical device offers multiple advantages for healthcare professionals:
- 24-hour Lipiodol resistance
- patented three-way stopcock with four connections offering possibility of “on-table mixing” where interventional radiologists have the possibility of remixing without disconnection from the micro-catheter, thus maximizing the safety during the intervention
- unique ready-to-use set with all devices in one set
- user-friendly, ergonomic and quick device set-up, improving cTACE procedures for physicians.
“Vectorio has been developed in collaboration with international interventional radiologists to match their medical needs for accurate, user-friendly and safe solution during cTACE procedures. The development of these image-guided procedures is a top priority for Guerbet’s Interventional franchise. We are committed to enhance liver cancer patients’ prognosis and quality of life worldwide” said Yves L’Epine, CEO of Guerbet.
Designed and manufactured in France, Vectorio’s commercial launch will start this fall in European countries, where Lipiodol Ultra Fluid is registered for cTACE. The Vectorio registration programme is also planned in other countries where cTACE is indicated for Lipiodol Ultra Fluid.










