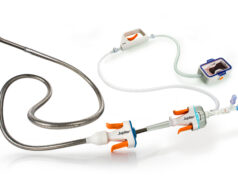
Flexible Stenting Solutions announced FDA investigational device exemption (IDE) approval for its FlexStent femoropopliteal self-expanding stent system on 2 February 2011.
The IDE allows Flexible Solutions to begin enrolment for the OPEN trial, a prospective, single-arm trial that will include up to 227 patients at up to 40 clinical sites in the United States. The study is scheduled to begin in the first quarter of 2011.
According to the company, the device is an atraumatic, highly durable, fatigue resistant stent with high radial strength. William A. Gray, principal investigator of OPEN, commented “This is a major milestone for the company and in combination with compelling early overseas data affords it the opportunity to further demonstrate the safety and efficacy of the product and ultimately lead to premarket approval here in the United States.”
Flexible Solutions previously announced that it has received FDA 510(k) clearance for the biliary FlexStent system. In Europe, CE mark approval has been granted for the biliary and femoropopliteal FlexStent systems, which include the same stent delivery system to be used in the United States clinical study.