Vivasure Medical has announced the successful enrolment of the first patient in the Frontier IV clinical study, a non-randomised multicentre international trial, designed to expand the indications of its proprietary PerQseal large arteriotomy closure technology. The patient was enrolled by Peter Crean (Blackrock Clinic, Dublin, Ireland).
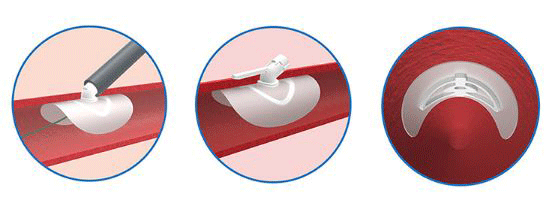
Large arteriotomies (12Fr) are vessel punctures created to facilitate endovascular procedures such as transcatheter aortic valve implantation (TAVI), endovascular aneurysm repair (EVAR), balloon valvuloplasty, and ventricular assist devices. A press release reports that PerQseal is the world’s first fully absorbable, patch-based large-bore percutaneous closure technology.
Christoph Naber (Contilia Heart and Vascular Centre, Essen, Germany), who is the TAVI principal investigator of Frontier IV, says: “Driven by clinical and economic outcomes data, percutaneous access-site management has become an increasingly important aspect of TAVI procedures. I strongly believe PerQseal, which is designed specifically to address large arteriotomies, will help improve outcomes for these patients.”
“We are very excited to begin the Frontier IV trial as the next phase in our commitment to build the clinical experience with PerQseal. A percutaneous approach has now become the gold standard for procedures such as TAVR and EVAR, driven by clinical outcomes data. As patient volumes increase, access site management and closure has become an increasingly important aspect of complication and cost reduction. The data from this trial will be used to support our goal of expanding the indication range of the PerQseal technology,” comments Gerard Brett, co-founder and CEO of Vivasure.