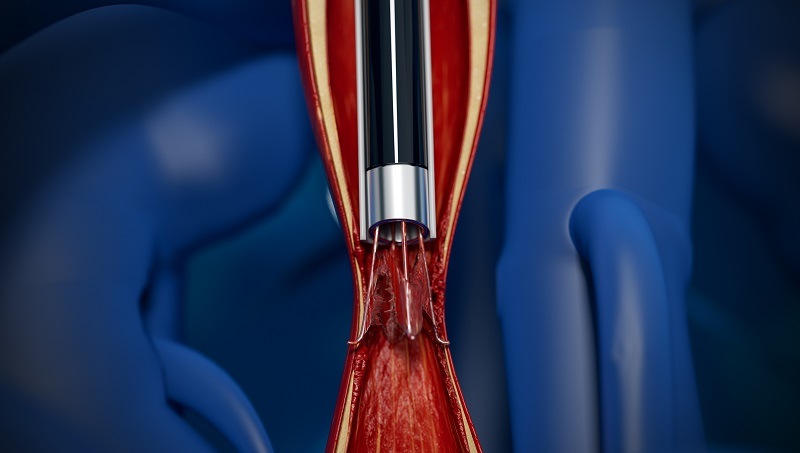
Royal Philips has announced US Food and Drug Administration (FDA) de novo clearance for the Philips inferior vena cava (IVC) filter removal laser sheath—CavaClear—to remove an IVC filter when previous methods of removal have failed.
Philips CavaClear IVC filter removal laser sheath is the first and only FDA-cleared solution for advanced IVC filter removal, the company said in a press release. Earlier in 2021, the FDA granted the device Breakthrough Device Designation. Laser has been clinically proven to provide a success rate over 99%, with low complication rates, the press release adds.
IVC filters are used to treat patients with venous thromboembolism, in which blood clots form in the deep veins of the leg and groin, and can travel through the circulatory system. They are placed in the inferior vena cava to capture blood clots from moving to the lungs.
Research has shown that IVC filters may have long-term complications, as the filters can fracture and travel through the bloodstream to other parts of the body. Other identified long-term risks associated with IVC filters include lower limb deep vein thrombosis and IVC occlusion. The FDA recommends that implanting physicians consider removing retrievable IVC filters as soon as they are no longer indicated.
Prior to CavaClear, limited options for removal existed if the filter became difficult to remove, according to Philips. Advanced retrieval tools and techniques are required if the IVC filter becomes embedded in the vasculature. Physicians previously had very few tools to remove the filter when complications occurred and until now there were no FDA-approved devices for this type of advanced removal.
“Today is a historic day. With the approval of CavaClear, physicians now have a device specifically geared remove chronically embedded IVC filters,” said Kush R Desai MD, associate professor of Radiology, Surgery, and Medicine, and director of deep venous interventions at Northwestern University Feinberg School of Medicine (Chicago, USA). “Backed by evidence, this technology can be applied to retrieve IVC filters that are no longer indicated, reducing potential clinical risk for patients and satisfying the FDA’s guidance to retrieve filters when they are no longer indicated.”
“With the FDA’s clearance of CavaClear more than one million patients and their physicians now have access to a safe, effective and efficient option for advanced IVC filter removal,” said Chris Landon, senior vice president and General Manager Image Guided Therapy Devices at Philips. “This clearance demonstrates the commitment of Philips to innovating procedures with physician collaboration to meet unmet needs that can have a critical impact on the lives of patients and their families.”










