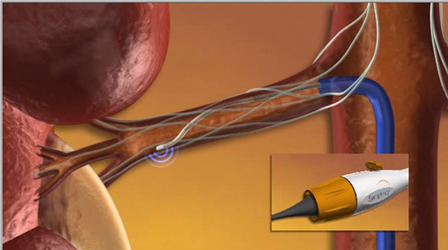
SYMPLICITY HTN-4 to potentially expand access for additional patients at risk for cardiovascular complications related to uncontrolled hypertension.
Medtronic has announced that the US Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) allowing the company to initiate SYMPLICITY HTN-4, the first randomised trial to investigate renal denervation for the treatment of moderate uncontrolled hypertension in US patients. The study will evaluate the Symplicity renal denervation system in patients with moderate uncontrolled hypertension (systolic blood pressure greater than or equal to 140 and less than 160mmHg, despite treatment with three or more antihypertensive medications of different classes). SYMPLICITY HTN-4, which enrolled its first patient at Duke University Medical Center, is Medtronic’s second randomised, controlled renal denervation clinical trial in the USA. The Symplicity renal denervation system is currently available only for investigational use in the USA.
“SYMPLICITY HTN-4 builds upon our rigorous clinical evaluation of the Symplicity renal denervation system designed to carefully and progressively develop the clinical evidence platform for the treatment of hypertension,” said Nina Goodheart, vice president, general manager, Renal Denervation, Medtronic. “We are excited to initiate this study, even while patient follow-up continues in our pivotal USA clinical trial for patients with uncontrolled hypertension who have systolic blood pressure at or above 160. The SYMPLICITY HTN-4 trial will allow us to potentially extend the benefits of renal denervation to patients with more moderate uncontrolled hypertension in the USA.”
“Over the past several years, the Symplicity renal denervation system has successfully treated patients outside the US with a more severe form of uncontrolled hypertension characterised by systolic blood pressures at or above 160mmHg,” explained David Kandzari, co-principal investigator of SYMPLICITY HTN-4 and chief scientific officer and director of Interventional Cardiology, Piedmont Heart Institute, Atlanta, USA. “However, many patients with uncontrolled hypertension have blood pressure between 140 and 160mmHg, making this also a very important group to study, since we know the risk for cardiovascular mortality and morbidity increases linearly for every millimetre of systolic blood pressure elevation above 140.”
“This highly-anticipated, robust study will serve a key role in evaluating renal denervation in these underserved patients with less severe hypertension so that we are able to understand what benefit this novel therapy may offer patients outside of the currently indicated patient population,” said Michael Weber, co-primary Investigator of SYMPLICITY HTN-4 and professor of Medicine, Division of Cardiovascular Medicine, at the SUNY Downstate College of Medicine in Brooklyn, USA.”