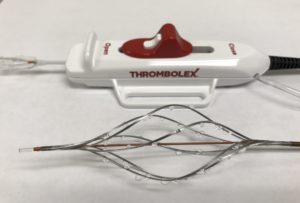
Thrombolex has announced that the company has received US Food and Drug Administration (FDA) clearance for the Bashir Endovascular Catheter – Short Basket (BEC S-B). The indication for use of the BEC S-B is for the controlled and selective infusion of physicianspecified fluids, including thrombolytics, into the peripheral vasculature.
Thrombolex provides an innovative line of endovascular catheters, which feature a unique combination of mechanical-pharmaco, expandable infusion basket for effective treatment of venous thromboembolic (VTE) conditions.
According to Thrombolex, the BEC S-B represents the third FDA 510k premarket pathway clearance achieved this year by the Company. This new medical device is part of the Company’s platform technology.
The Bashir line of interventional catheters are the first interventional catheters to rapidly create multiple cross-sectional channels to allow the patient’s own endogenous lytics to immediately begin to flow into the culprit thrombus. The treatment is then enhanced by infusing small amounts of exogenous thrombolytics directly into the culprit thrombus, the combination of which accelerates the dissolution of the clot burden.
The Bashir Endovascular Catheter (BEC) and the Bashir N-X Endovascular Catheter (BEC N-X) previously received FDA 510k premarket pathway clearance earlier this year. The BEC is cleared for the controlled and selective infusion of physicianspecified fluids, including thrombolytics, into the peripheral vasculature. The BEC N-X is a device intended for the localised infusion of physician specified fluids, into the peripheral vasculature, including the pulmonary arteries.
The company has advised that the BEC S-B will be introduced into the USA through a controlled roll-out at hospitals with established VTE treatment protocols.










