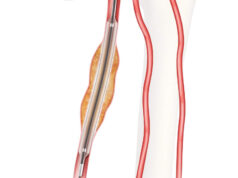
Cook Medical has initiated a Class I recall of its Beacon Tip 5Fr angiographic catheter.
This follows reports of tip separation that could result in serious injury or death. The recall, identified as the most serious type by the US Food and Drug Administration (FDA), involves the removal of affected devices from clinical and commercial use.
In its notification to customers, Cook advised immediate examination of inventory to identify and quarantine any unused affected devices. Distribution and use of the affected catheters must cease immediately. The manufacturer also stressed that the recall information should be communicated throughout relevant departments and to any third parties who may have received the devices.
Cook Medical initiated the recall after field complaints revealed that catheter tips were separating both before and during patient procedures. Tip separation poses significant clinical risks, including catheter fragmentation and embolization, which may lead to severe complications such as sepsis, vessel perforation, thrombosis, embolism, cardiac arrhythmia, or death.
To date, three serious injuries linked to this issue have been reported, with no associated deaths.
Beacon Tip catheters are used by trained physicians to facilitate angiographic procedures, which provide imaging of blood vessels. These devices are commonly employed in combination with vascular access sheaths and guidewires using standard interventional techniques.
Healthcare providers and consumers in the USA are encouraged to report any adverse reactions or quality concerns related to this device to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.