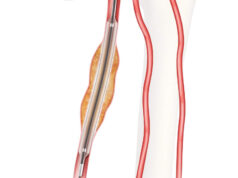
Cook Medical has introduced the 140mm-length Zilver PTX drug-eluting peripheral stent in both 6 and 7mm diameters in the USA. The longer length comes after an expanded indication approval by FDA to treat total lesion lengths up to 300mm per patient. In addition, the product also received an extended shelf life of two years by the FDA.
The 140-mm length stents will enable physicians when applicable to treat lesions up to 270-mm with only two stents. In addition to this benefit, the stent has been shown to cut reinterventions by nearly half in 5 years, compared to a combination of bare-metal Zilver stents and PTA.
“We’re happy to continually broaden the Zilver PTX product offering, giving physicians more options when treating superficial femoral artery disease based on the individual patient need,” said Mark Breedlove, vice president of Cook Medical’s vascular division.
The device is the world’s first drug-eluting stent for treating peripheral arterial disease (PAD) in the superficial femoral artery (SFA) and is the only drug-eluting SFA stent with five-year published data.