 Bluegrass Vascular has announced receiving CE mark approval for the Surfacer Inside-Out access catheter system, indicated for obtaining central venous access to facilitate catheter insertion into the central venous system via a novel “Inside-Out” approach. The company is also launching a limited commercial sale of the system.
Bluegrass Vascular has announced receiving CE mark approval for the Surfacer Inside-Out access catheter system, indicated for obtaining central venous access to facilitate catheter insertion into the central venous system via a novel “Inside-Out” approach. The company is also launching a limited commercial sale of the system.
“The launch of The Surfacer system in Europe brings a much-needed solution to a growing population of patients requiring vascular access for vital treatments and who currently have limited options due to obstructed upper body access,” stated Gürkan Sengölge, associate professor of Medicine, Nephrology and Intensive Care Medicine, Medical University of Vienna, Austria.
“I was very pleased with my first commercial use of the Surfacer system. No other device offers such an innovative ‘Inside-Out’ method for restoring access and preserving options in patients with chronically occluded veins. Based on my initial experience, I am confident that The Surfacer system will change the standard of care going forward.”
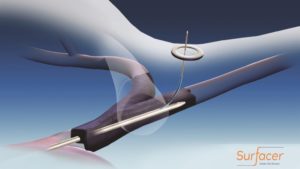
The Surfacer system is designed to reliably, efficiently and repeatedly gain central venous access by being inserted through the femoral vein and being navigating up through the patient’s venous system with an exit point in the right internal jugular vein, the optimal location for placing a central venous catheter. This proprietary inside-out approach allows for the placement and maturation of permanent arteriovenous access options that are associated with improved patient outcomes and reduced cost of care for both hospitals and haemodialysis providers.
“Multiple central venous occlusions can cause significant long-term morbidity,” explained John Gurley, interventional cardiologist, University of Kentucky Medical Center, Bluegrass Vascular founder and developer of the inside-out method. “The novel ‘Inside-Out’ approach is an innovative and simple method that restores and preserves right-sided venous access despite chronic occlusion and retains the viability of other existing central veins.”
“This is a major milestone both for our company and for patients with occlusive disease,” stated Gabriele Niederauer, CEO and president of Bluegrass Vascular. “After successful use of our device in initial commercial cases and previously under Germany’s Compassionate Use Programme, we are eager to commercialise the system in Europe and meet the clinical demand from physicians treating patients with occluded central veins.”
Bluegrass Vascular has launched the Surfacer system in limited markets, including the UK, Germany, Austria, Italy, and the Benelux region. The company will also commence enrolment in the SAVE (the Surfacer system to facilitate access in venous occlusions) study, an observational, post-market registry for patients with limited or diminishing upper body venous access or pathology impeding standard access methods.













