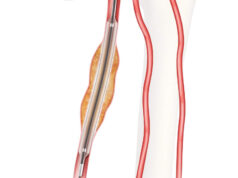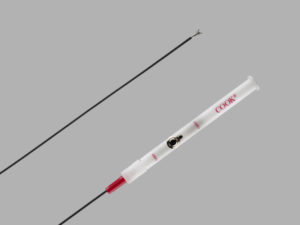
The Transluminal Biliary Biopsy Forceps Set (BBFS) and a new 40-centimetre delivery system line extension of the existing Zilver 635 Biliary stent (both from Cook Medical) are now commercially available in the USA.
“We are excited to make these products available to physicians to provide more treatment options for biliary duct procedures. This continues our efforts to bring products to physicians that fill the unmet clinical needs for patients,” comments Mike Williams, director of Cook Medical’s vascular programmes.
According to a company press release, the Transluminal Biliary Biopsy Forceps Set is designed to facilitate percutaneous acquisition of accurate tissue samples for biopsy. Cook Medical go on to state that biopsy samples obtained during biliary procedures “must be sufficient and precise to reflect accurate diagnosis”. BBFS was developed to include an introduction system coupled with a “cup and jaw” forceps design to retrieve biopsy specimens. Supporting the use of the BBFS design, a study published in Cardiovascular and Interventional Radiology reported a 94.2% diagnostic accuracy, with appropriate technique, when evaluating malignant biliary strictures using BBFS.
The Zilver 635 Biliary Stent is designed for cases of abnormal constrictions of the biliary tree (biliary neoplasms). The product helps to restore the natural flow of the biliary duct system, Cook Medical claims in a statement on the product launch. The flexible stent is also resistant to shortening, helping to provide precise placement. Having a 40-centimetre long delivery system permits ease of access for placement of the stent in biliary neoplasms.