BD (Becton, Dickinson and Company) has announced the launch and 510(k) clearance from the US Food and Drug Administration (FDA) of the Crosser iQ chronic total occlusion (CTO) recanalisation system, which is designed to cross peripheral artery CTOs intraluminally.
The Crosser iQ ultrasonic CTO device was developed to help improve CTO crossing predictability to aid clinicians treating these challenging lesions—the CTO crossing device also pairs the Crosser iQ ultrasonic CTO device with the BD recanalization system and the Recon support catheter. The new system utilizes proprietary technology that automatically adjusts the crossing power output to help stabilize the distal tip of the device to assist in crossing through the entire length of the CTO intraluminally.
The BD recanalization system and Crosser iQ ultrasonic CTO device is indicated to facilitate the intraluminal placement of conventional guidewires beyond peripheral artery CTOs, the company claims. The Crosser iQ ultrasonic CTO device is contraindicated for use in carotid arteries.
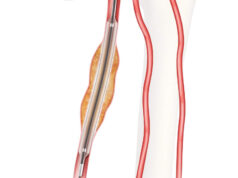
According to the company, the device utilizes a unique mechanism of action that is specifically designed to vibrate against peripheral arterial CTOs creating an intraluminal channel to facilitate the placement of conventional guidewires.
During the procedure, power from the BD recanalization system is converted into ultrasonic vibrational energy causing microbubbles to expand and implode at the tip of the Crosser iQ ultrasonic CTO device. This breaks the internal structure of the plaque and erodes the solid surface of the CTO, allowing the physician to automatically and selectively ablate plaque, while remaining atraumatic to elastic tissue, advised BD.