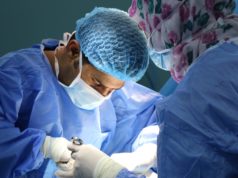
Baird Medical Devices, a microwave ablation medical device developer and provider in China and the USA, has recently announced that it has performed its first thyroid microwave ablation procedure in the USA since obtaining registration clearance from the US Food and Drug Administration (FDA) on November 22, 2023.
Baird Medical Devices, a microwave ablation medical device developer and provider in China and the USA, has recently announced that it has performed its first thyroid microwave ablation procedure in the USA since obtaining registration clearance from the US Food and Drug Administration (FDA) on November 22, 2023.
The minimally invasive procedure was performed on 12 January 2024 at Utah Endocrinology Associates by interventional endocrinologist Alireza Falahati, marking a significant advancement in thyroid nodule management, a press release states. The emergent microwave ablation procedure for thyroid nodules offers patients an alternative to traditional excision surgery, enabling swifter recovery, a reduction of scar tissue in the neck and chest area, and greater efficiency for physicians.
Falahati expressed his enthusiasm for the new treatment option: “The success of this procedure with Baird Medical’s ablation needle represents a leap forward in our ability to treat thyroid nodules effectively. I look forward to performing many more similar procedures in the near future.”
Thyroid nodules occur in 30–50% of the adult population, with a rise of incidence as people age, and are more common with women than men. Microwave ablation procedures utilise a small needle to safely deliver therapeutic microwave energy directly to the nodule being treated while sparing the surrounding healthy tissue and preserving the function of the thyroid, thereby eliminating hormone replacement therapy affiliated with surgical approaches.
“We congratulate Falahati and his first patient for breaking new ground with us in this important therapeutic area,” said Haimei Wu, founder and CEO of Baird Medical. “This procedure was conducted less than 60 days after the FDA’s clearance of our advanced technology for the market in the USA. We are excited about the opportunities this offers to millions of patients with unmet needs in the future.”
Due to recent technological advancements and increasing awareness, there has been a rapid increase in the number of minimally invasive, thermal ablation procedures performed. In September 2023, the American Thyroid Association (ATA) published their recommended guidelines for the safe implementation and adoption thermal ablation procedures to treatment of thyroid nodules, suggestive of the increased adoption of the procedure.