willdate
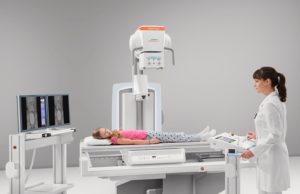
GE HealthCare’s Allia Moveo gains FDA and CE mark approvals
GE HealthCare has announced that its Allia Moveo image-guidance platform has received US Food and Drug Administration (FDA) 510(k) clearance and CE mark.
The latest...
SonoMotion announces US FDA clearance for its Break Wave lithotripsy device
SonoMotion, a venture-backed medical device company developing non-invasive solutions for kidney stones, announced today that it received US Food and Drug Administration (FDA) 510(k)...
Clinical trial of covered stent graft for dialysis access dysfunction completes...
Solaris Endovascular has announced that the DEScover clinical trial has successfully completed enrolment.
DEScover is a prospective, multicentre clinical study evaluating the Solaris DE sirolimus-eluting,...
Haemonetics acquires vessel closure specialist Vivasure Medical
Haemonetics Corporation today announced the acquisition of Vivasure Medical, a Galway, Ireland-based company developing next-generation technology for percutaneous vessel closure.
Vivasure's PerQseal Elite system uses...
First patient treated with A3-Shield system in PRINCIPIIS FIH trial
Angiolutions has announced that the first patient has been successfully treated with its investigational A3-Shield system in the PRINCIPIIS FIH trial, a first-in-human (FIH)...
Akura Medical closes series C financing
Akura Medical has secured a US$53 million first close in series C financing, with the funds to be used to support development activities for...
Siemens Healthineers launches AI-powered imaging system, Optiq AI
Siemens Healthineers has launched its new imaging chain, Optiq AI, an artificial intelligence (AI)-powered system that is designed to deliver high quality low-dose images...
Vascular complications uncommon in study of latest-generation arterial closure device
No major vascular complications were seen in the ELITE trial using the latest-generation PerQseal Elite (Vivasure Medical), a patch-based, resorbable, sutureless vascular device for...
TCT 2025: Trials find comparable outcomes for IVL alternatives in calcified...
Cutting balloon angioplasty and the use of a super-high-pressure non-compliant balloon have been shown to be non-inferior to intravascular lithotripsy (IVL) in separate randomised...
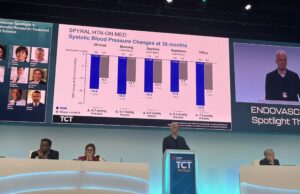
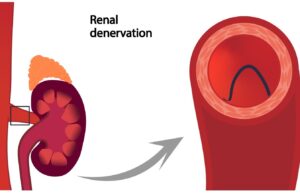
TCT 2025: Three-year results demonstrate “durable” impact of radiofrequency renal denervation
Medtronic has announced new, long-term results from its final report of the SPYRAL HTN-ON MED randomised clinical trial, showing that patients treated with the...
Jupiter Endovascular secures funds to continue development of TFX platform
Jupiter Endovascular has closed a series B financing round, surpassing its US$40 million target, with proceeds to be used to complete the ongoing SPIRARE...
VASBI panel debates suitability of randomised trials in vascular access
Optimal trial design for vascular access studies went under the microscope at the Vascular Access Society of Britain and Ireland (VASBI) 2025 annual scientific...
Study highlights safety and efficacy of Aperto OTW DCB in treating...
Cardionovum has announced that the APERTO 600 study, evaluating the efficacy of the Aperto over-the-wire (OTW) drug-coated balloon (DCB) in vascular access occlusion management,...
Xeltis poised to commercialise aXess vascular access conduit following pivotal trial...
Xeltis has announced clinical data from the aXess EU pivotal trial the prospective study investigating the patency, safety, and performance of its restorative vascular...
SYMPHONY-PE trial demonstrates positive efficacy, efficiency, and safety results
Imperative Care has announced efficacy and safety results from the pivotal SYMPHONY-PE trial evaluating the company’s Symphony thrombectomy system in the treatment of acute...
CORE-MD consortium announces recommendations on clinical assessment of high-risk medical devices
The CORE-MD—Coordinating Research and Evidence for high-risk Medical Devices—consortium has published new consensus recommendations in The Lancet Regional Health Europe, setting out scientifically robust...
Symplicity Spyral renal denervation system gains Japanese PDMA approval
Medtronic has announced it received approval in Japan from the Pharmaceuticals and Medical Devices Agency (PMDA) for its Symplicity Spyral renal denervation system for...
Paradise ultrasound renal denervation system gets approved for use in Japan
Recor Medical has announced that the Paradise ultrasound renal denervation system has received approval for manufacturing and marketing in Japan for the treatment of...
US blood pressure guidelines bump up recommendation for renal denervation
New guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA) for the prevention and management of high blood pressure...
Instylla’s Embrace HES gains US FDA premarket approval
Instylla has announced premarket approval (PMA) from the US Food and Drug Administration (FDA) for the company’s flagship product Embrace Hydrogel Embolic System (HES)....
CMS recommends coverage for renal denervation for hypertension
The US Centres for Medicare & Medicaid Services (CMS) has issued its proposed National Coverage Determination (NCD) on renal denervation, recommending coverage for the...
US patent issued for algorithm to predict renal denervation response
Geneticure has announced that the US Patent and Trademark Office has granted a patent for a genetic-based weighted algorithm model for predicting response to...
First patient enrolled in RECOVER-AV trial of AlphaVac F18 system for...
AngioDynamics has announced the first patient has been enrolled in the RECOVER-AV clinical trial, a prospective, multicentre, international, single-arm study evaluating the AlphaVac multipurpose...
New medical device reporting requirements come into effect in the UK
New regulations have come into effect in the UK which place a greater emphasis on medical device manufacturers to monitor the safety and performance...
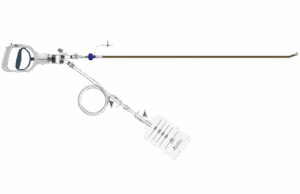
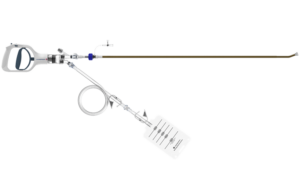
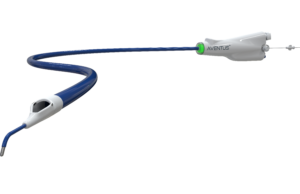
Aventus thrombectomy system gains FDA clearance for PE treatment
Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded...
PerQseal Elite vascular closure system gains CE mark
Vivasure Medical has announced European CE mark approval of the PerQseal Elite vascular closure system, a sutureless and fully bioresorbable large-bore vessel closure device....
Adept Medical updates its IR platform and overhead arm support ranges
Adept Medical has announced two updated product launches, coinciding with the Society of Interventional Radiology’s 50th annual scientific meeting (29 March–2 April, Nashville, USA).
This...
One-year WAVE trial results show patency advantage for Wrapsody versus PTA
Merit Medical Systems has announced that the six-month results from the randomised arm of the Wrapsody arteriovenous access efficacy—WAVE—trial are scheduled for publication in...
Stereotaxis seeks to broaden use of its robotic magnetic navigation system...
Stereotaxis has announced a US Food and Drug Administration (FDA) regulatory submission for the first robotically navigated catheter designed to expand usage of robotic...
DeepQure advances clinical trials of its HyperQure extravascular renal denervation system
DeepQure has announced progress of clinical trials of its extravascular renal denervation device, HyperQure.
The company is actively conducting clinical trials in both South Korea...
Boston Scientific to acquire renal denervation technology developer SoniVie
Boston Scientific has entered into a definitive agreement to acquire SoniVie Ltd, a privately held medical device company that has developed the TIVUS intravascular...
Challenges of 21st century aortic education, innovation and evidence get top...
Critical challenges in open and endovascular treatment of aortic disease will be brought into focus when world leaders in cardiac, aortic, and vascular therapies...
American Society of Echocardiography releases new guideline on ultrasound-guided vascular access
A new guideline from the American Society of Echocardiography (ASE) aims to provide more detail for clinicians performing ultrasound-guided vascular cannulation.
Guidelines for Performing Ultrasound-Guided...
CMS launches review of coverage for renal denervation
The Centers for Medicare & Medicaid Services (CMS) is opening a national coverage analysis (NCA) on renal denervation, with a view to reviewing and...
APEX-AV trial results of AlphaVac PE system published in JSCAI
AngioDynamics has announced the publication of the results from the Acute Pulmonary Embolism Extraction Trial with the AlphaVac system—APEX-AV—in the Journal of the Society...
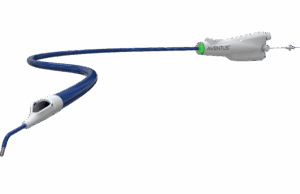
AVENTUS trial of thrombectomy system for PE treatment completes enrolment
Inquis Medical has announced completion of patient enrolment in its AVENTUS clinical trial, a pivotal investigational device exemption (IDE) trial evaluating the safety and...
Gore receives CE mark for lower profile Viabahn VBX endoprosthesis
Gore has announced recent CE mark of a lower profile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft).
Medical Device Regulation (MDR) approval of this...
Boston Scientific to acquire IVL developer Bolt Medical
Boston Scientific has announced it has entered into a definitive agreement to acquire Bolt Medical, the developer of an intravascular lithotripsy (IVL) advanced laser-based...
Xeltis completes enrolment in EU pivotal trial
Xeltis has announced the completion of enrolment in its EU pivotal trial for aXess, its restorative vascular access conduit, in adults with end-stage renal...
FastWave issued sixth utility patent for its IVL portfolio
FastWave Medical has announced the issuance of its sixth utility patent by the US Patent and Trademark Office (USPTO), the fourth patent the company...
US FDA approves IDE application for Katana thrombectomy system
The US Food and Drug Administration (FDA) has approved Akura Medical’s investigational device exemption (IDE) application to initiate the QUADRA-PE study evaluating the Katana...
First patient enrolled in study of integrated laser atherectomy and IVL...
Royal Philips has announced enrolment of the first patient in the US THOR IDE clinical trial, which will study an innovative combined laser atherectomy...
TCT 2024: Two-year SPYRAL HTN-ON MED show “consistent” effect of renal...
Medtronic has announced new, long-term data from the SPYRAL HTN-ON MED clinical trial that showed subjects who underwent radiofrequency renal denervation with the Symplicity...
First US cases performed with Vertex PE system in SPIRARE II...
Jupiter Endovascular has announced that the first US patient has been treated in the SPIRARE II US pivotal study of the Vertex pulmonary embolectomy...
First cases completed in SPIRARE I trial with Vertex system
Jupiter Endovascular has announced that the first two patients have been treated in SPIRARE I, a multicentre study of the Vertex pulmonary embolectomy system...
BioCardia submits 510(k) approval for Morph DNA steerable introducer sheath family
BioCardia has submitted a 510(k) for approval of its patented Morph DNA steerable introducer sheath to the US Food and Drug Administration (FDA).
This product...
NMPA approves SyMap renal denervation system for treatment of uncontrolled hypertension
China’s National Medical Products Administration (NMPA) has granted a Class III Medical Devices Certificate of Registration for the msRDN radiofrequency renal denervation system (SyMap...
First patients treated in US GPS study of Paradise renal denervation...
The first patients in the USA have been treated in Recor Medical’s Global Paradise System US Post Approval Study (US GPS), a real-world study...
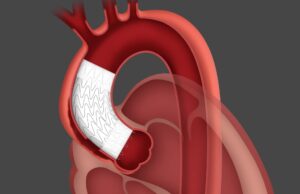
Abbott’s Esprit BTK scaffold system given US FDA approval for CLTI...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the Esprit BTK everolimus-eluting resorbable scaffold system (Esprit BTK system), a...
Fastwave Medical issued fourth utility patent for laser-IVL platform
FastWave Medical has announced the issuance of its fourth utility patent by the United States Patent and Trademark Office (USPTO) for its laser-intravascular lithotripsy...
Johnson & Johnson to acquire Shockwave Medical
Johnson & Johnson is to acquire Shockwave Medical, it has been announced today.
Under the terms of the transaction, Johnson & Johnson will acquire all...
Debates to unpick controversies in aortic repair at CX 2024
Debates will shed new light on controversies in the treatment of aortic disease at the 2024 Charing Cross (CX) Symposium, alongside practical sessions with...
Study of TIVUS renal denervation system for hypertension treatment completes enrolment
The REDUCED-1 study, a US Food and Drug Administration (FDA) investigational device exemption (IDE)-approved pilot study of the treatment of hypertension with the...
Radiofrequency renal denervation meets cost efficiency threshold in value analysis
An analysis of data from the SPYRAL HTN-ON MED trial of the Symplicity Spyral (Medtronic) radiofrequency denervation system for the treatment of uncontrolled hypertension...
FastWave Medical announces completion of first procedures in study of novel...
FastWave Medical has announced the successful completion of enrolment for its first-in-human (FIH) study with the company's peripheral intravascular lithotripsy (IVL) technology.
The prospective, single-arm...
US FDA approves ENGULF US pivotal trial of Hēlo thrombectomy system
Endovascular Engineering (E2) has announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for its ENGULF US pivotal trial.
The study will...
GE HealthCare to acquire AI-imaging specialist
GE HealthCare has entered into an agreement to acquire MIM Software, a provider of artificial intelligence (AI)-enabled image analysis and workflow tools across multiple...
First patient treated in ARISE II study of Gore ascending stent...
Gore has announced the first patient implantation of the Gore ascending stent graft in the ARISE II trial, describing this as an exciting step...
Microbot Medical and Corewell Health announce telerobotics collaboration
Microbot Medical, developer of the Liberty endovascular robotic surgical system, has entered into a collaboration agreement with Corewell Health.
The objective of the collaboration, which...
Kaneka Corporation acquires Japan Medical Device Technology Co
Kaneka Corporation has acquired all shares of Japan Medical Device Technology Co and has made it a wholly owned subsidiary.
Kaneka manufactures and sells endovascular...
Medtronic’s Symplicity Spyral renal denervation system granted US FDA approval for...
Medtronic has announced approval from the USA Food and Drug Administration (FDA) for the Symplicity Spyral renal denervation (RDN) system for the treatment of...
PATCH clinical study of PerQseal vascular closure system reaches 100-patient milestone
Vivasure Medical has enrolled the 100th patient in its PATCH clinical study, a multicentre, single-arm, pivotal study evaluating the safety and efficacy of the...
Scott Drake appointed as Cordis CEO
Cordis has announced that its board of directors has appointed Scott Drake as chief executive officer (CEO) effective immediately. Drake most recently held the...
Recor Medical gains first US FDA renal denervation approval
Recor Medical has become the first company in the USA to have a device-based therapy approved for the treatment of hypertension, after it was...
E2 shares positive initial results from use of Hēlo system for...
Endovascular Engineering (E2) has announced positive initial results from its ENGULF study, demonstrating successful outcomes among the first 15 patients evaluated in the trial...
TCT 2023: First presentation of complete patient-level dataset on paclitaxel and...
Data from a patient-level meta-analysis—a factor in the US Food and Drug Administration’s (FDA) recent change of position on the use of paclitaxel-coated devices...
Multicentre study points to favourable safety and efficacy of radial access...
New research examining the safety and efficacy of using radial access for peripheral artery interventions has found that radial access allows early ambulation and...
MedAlliance acquired by Cordis
MedAlliance has been acquired by Cordis for a 2022 investment of US$35 million and a 2023 upfront closing payment of US$200 million, together with...
Nick West appointed to associate CMO role at Shockwave Medical
Shockwave Medical has announced that Nick West has joined the company in the role of associate chief medical officer (CMO). He will report to...
UK MHRA adds capacity for medical device certification
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has designated three new approved bodies to increase the country’s capacity to certify medical devices.
TÜV...
SCAI: FDA panel vote “encouraging” for advance of renal denervation
The Society for Cardiovascular Angiography & Interventions (SCAI) has welcomed the review of scientific data related to the premarket approval applications for two renal...
ClotTriever “makes fast, safe and efficient thrombectomy possible”
This advertorial is sponsored by Inari Medical
“We need to eliminate symptoms as fast as possible—it is not OK just to make things a little...
Timeline: Key milestones in the paclitaxel story
The release of a letter from the US Food and Drug Administration (FDA) concerning the use of paclitaxel coated devices for the peripheral arterial...
Approval granted to commence study of renal pelvis denervation system
Verve Medical has received approval from the US Food and Drug Administration (FDA) to initiate its Natural Orifice via renal pelvis denervation (NOVEL-Denervation) pivotal...
Long-awaited US FDA update finds data do not support excess mortality...
In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA) communicates that the risk of mortality associated...
US FDA approves study of ZFEN+ for the treatment of aortic...
The US Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Zenith...
US FDA seeks to “modernise” clinical trials with new draft guidance
The US Food and Drug Administration (FDA) has released draft guidance with updated recommendations for good clinical practices (GCPs) aimed at modernising the design...
Amazon executive joins Medtronic to spearhead development in robotics and implantables
Medtronic has announced that Ken Washington has been appointed chief technology and innovation officer.
Washington joins Medtronic from Amazon where he served as vice president...
Proprietary antioxidant complex shown to safeguard DNA from radiation exposure during...
Cora Therapeutics has announced the presentation of the results of a clinical trial at the Canadian Association of Interventional Radiologists (CAIR) annual meeting (25–27...
SCAI 2023: Mechanical thrombectomy improves symptoms and quality of life in...
Six-month outcomes from FLASH registry have shown that patients with pulmonary embolism who were treated with mechanical thrombectomy showed significant improvement in symptoms, quality...
EuroPCR 2023: Imaging technology reduces contrast use during PCI
The use of an image-guidance system that overlays angiographic images on live fluoroscopic images has been shown to reduce the amount of contrast used...
Handheld ECG device scoops CX 2023 Innovation Showcase prize
Judges of the CX 2023 Dragon’s Den-style contest—the finale of the Charing Cross (CX) Symposium (25–27 April, London, UK) Innovation Showcase programme—described the field...
EU ministers approve changes to MDR transition timetable
The European Union’s Council of Ministers has today adopted a resolution to extend the deadline for the certification of medical devices under the Medical...
ACC 2023: Large bore mechanical thrombectomy reduces adverse outcomes in high-risk...
Large bore mechanical thrombectomy with the FlowTriever system (Inari Medical) in patients with high-risk pulmonary embolism (PE) was associated with a significantly lower occurrence...
Renal denervation “can reach new shores”
The publication of a consensus statement from the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions...
Xeltis secures funding to progress clinical trials
Xeltis has raised €32 million in a Series D2 equity fundraise, backed by a syndicate of current and new investors, which the company says...
ESC and EAPCI publish renal denervation consensus statement
Renal denervation represents another treatment option in patients with uncontrolled resistant hypertension and may be used in selected patients deemed intolerant to antihypertensive drugs.
These...
Funding secured to advance development of Pulse IVL system
AVS has announced that it raised US$20 million in Series B financing, which the company says will accelerate clinical trial timelines for its device...
ReCor appoints Lara Barghout to lead commercialisation of Paradise renal denervation...
ReCor Medical and its parent company Otsuka Medical Devices have announced the appointment of Lara Barghout as president and chief executive officer of ReCor.
Barghout...
Corindus rebrands to Siemens Healthineers Endovascular Robotics
Corindus has been rebranded to Siemens Healthineers Endovascular Robotics and will sit as a dedicated business within the Advanced Therapies area of Siemens Healthineers,...
Efemoral Medical closes financing round to fund ongoing study of its...
Efemoral Medical has today announced the closing of its US$4.9 million preferred Series A1 round. Supported by existing investors as well as an experienced...
EU Commission to propose delay to MDR implementation
Europe’s health commissioner, Stella Kyriakides, has announced that proposals to extend the transition period for the implementation of the European Union’s (EU) Medical Device...
TCT 2022 hears “important data” from renal denervation trials
Findings from the RADIANCE II randomised pivotal trial, presented at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA), “confirm that ultrasound...
CX Aortic Vienna: Cardiac and vascular surgeons collaborate at the vanguard...
CX Aortic Vienna returns in October for its third edition (24–26 October, Digital), bringing together world-leading specialists from the cardiac and vascular fields to...
Ultrasound renal denervation meets primary efficacy endpoint in RADIANCE II study
ReCor Medical and Otsuka Medical Devices have announced that the RADIANCE II US Food and Drug Administration (FDA) investigational device exemption (IDE) pivotal trial...
Medtronic gains FDA approval for IN.PACT 018 DCB
Medtronic has announced approval from the US Food and Drug Administration (FDA) for the IN.PACT 018 paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheter, a...
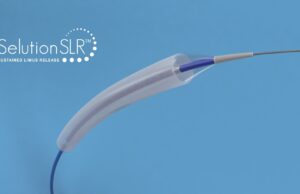
FDA grants IDE approval for Selution SLR drug-eluting balloon
MedAlliance’s Selution SLR drug-eluting balloon (DEB) has received investigational device exemption (IDE) approval from the US Food and Drug Administration, making it the first...
EuroPCR 2022: Studies underscore potential of renal denervation as an adjunctive...
Late-breaking trial data presented at EuroPCR 2022 (17–20 May, Paris, France) underscore the potential of renal denervation as an adjunctive therapy to treat hypertension,...
First patients enrolled in IliCo study of iCover stent
iVascular has announced the initiation of its first trial with the new generation covered stent iCover—the iliCo study. The study has the objective of...
First patient enrolled in PEERLESS study of FlowTriever system
Inari Medical has announced that the first patient has been enrolled in PEERLESS prospective, randomised controlled trial (RCT) comparing the outcomes of patients with...
Philips expands haemodynamic assessment in its Lumify handheld ultrasound system
Royal Philips has announced an update to its handheld ultrasound platform—Lumify—adding Pulse Wave Doppler technology to expand the haemodynamic assessment and measurement capabilities of...
FDA clears CavaClear IVC filter removal laser sheath
Royal Philips has announced US Food and Drug Administration (FDA) de novo clearance for the Philips inferior vena cava (IVC) filter removal laser sheath—CavaClear—to...
CSI recalls Wirion embolic protection device
Cardiovascular Systems has initiated a voluntary recall of unused Wirion embolic protection systems due to complaints of filter breakage during retrieval.
Wirion is a distal...
Medtronic announces 2045 net zero emissions ambition to combat climate change
Medtronic has announced its ambition to achieve net zero carbon emissions by fiscal year 2045 across its operations and value chain to accelerate efforts...
TCT 2021: Randomised trials show “consistent” reduction in blood pressure through...
Randomised placebo-controlled trials show consistently that renal denervation provides significant reduction in ambulatory and office blood pressure, the findings of a systematic review and...
TCT 2021: Six-month RADIANCE-HTN TRIO results “open a window” for renal...
Six-month outcomes from the randomised RADIANCE-HTN TRIO trial, comparing endovascular ultrasound renal denervation to a sham procedure for treatment-resistant hypertension, demonstrate the additional effects...
Siemens launches Luminos Impulse fluoroscopy system
Siemens Healthineers has launched the Luminos Impulse fluoroscopy system, including features such as a seamless imaging chain, comprehensive dose optimisation, cybersecurity features, and detector-sharing...
Gore’s lower profile delivery Viabahn endoprosthesis gets EMEA launch
Gore has announced the EMEA launch of the lower profile, large diameter Viabahn endoprosthesis with Propaten bioactive surface.
The device enhancements build on a market-leading...
Renal denervation: The most advanced and efficacious interventional approach to treat...
Following the release of the European Society of Hypertension’s (ESH’s) updated position paper on renal denervation Konstantinos Tsioufis, professor of Cardiology at the University...
Interventional News’ top 10 most popular stories of July 2021
July’s top 10 features a video interview with Jonathan G Moss (Institute of Cardiovascular and Medical Sciences, Glasgow, UK), chief investigator of the CAVA...
MDR comes into effect across EU
The European Union (EU) Medical Devices Regulation (MDR) takes effect from today (26 May 2021).
The Regulation revises quality and safety standards and the range...
ACC.21: VOYAGER PAD analysis shows reduced risk of ischaemic events in...
Rivaroxaban (Xarelto, Bayer/Janssen), in addition to low-dose aspirin, should be considered as an adjunctive therapy after lower extremity revascularisation to reduce first and subsequent...
Cardiovascular Systems announces first uses of Wirion embolic protection system
Cardiovascular Systems has announced that the first patients in the USA have been treated with the Wirion embolic protection system. Wirion is a distal...
ControlRad Select imaging system gains FDA market clearance
ControlRad has announced US Food and Drug Administration (FDA) 510(k) clearance to market ControlRad Select, a technology that utilises proprietary semi-transparent filters, a user-interface...
Terumo reaches agreement to acquire predictive analytics specialist Health Outcomes Sciences
Terumo Corporation has signed a definitive agreement to acquire all assets of Health Outcomes Sciences, a specialist in predictive analytics and clinical decision support...
Comparative study reports favourable outcomes for VasoStat haemostasis device
Forge Medical has announced the publication of results of an investigator-initiated randomised trial comparing its VasoStat haemostasis device to the TR Band (Terumo Medical)...
Comparative study reports favourable outcomes for VasoStat haemostasis device
Forge Medical has announced the publication of results of an investigator-initiated randomised trial comparing its VasoStat haemostasis device to the TR Band (Terumo Medical)...
Corindus announces global launch of technIQ procedural automation series for CorPath...
Corindus has announced today global launch of a new set of automated robotic movements in the technIQ Series designed for the CorPath GRX system....
Boston Scientific agrees to divest BTG Specialty Pharmaceuticals business
Boston Scientific has announced that it has entered into a definitive agreement with Stark International and SERB SAS, affiliates of the European specialty pharmaceutical...
Sinomed announces positive safety and efficacy data from PIONEER III study
Sinomed has announced the presentation of data from its inter-continental PIONEER III study comparing the safety and efficacy of the Supreme HT (healing-targeted) drug-eluting...
Philips launches latest version of its Azurion imaging platform
Royal Philips has announced the next-generation of its Azurion image-guided therapy platform, designed to improve the quality and efficiency of interventional procedures. The Azurion...
RSNA grant enables the establishment of an IR service in Tanzania
“There is a general misconception that you cannot do interventional radiology (IR) in a resource limited setting,” says Fabian Laage Gaupp, IR resident at...
Six step plan at the heart of Singapore General Hospital’s COVID-19...
The COVID-19 pandemic will have a deep impact on the future of healthcare according to Tan Bien Soo, Singapore General Hospital (Singapore). He tells...
FDA clears Transit Scientific’s XO Score PTA scoring sheath platform
Transit Scientific has received US Food and Drug Administration (FDA) clearance for the XO Score percutaneous transluminal angioplasty (PTA) scoring sheath platform for use...
IR community urged to plan for COVID-19 cases
Interventional radiologists from Singapore have shared their strategies for the preparation of IR services to cope with COVID-19 patients, emphasising the need for clear...