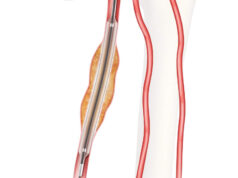
Getinge and Cook Medical today announced an exclusive sales and distribution agreement for the iCast covered stent system, which recently received US Food and Drug Administration premarket approval for the treatment of symptomatic iliac arterial occlusive disease.
Cook Medical will assume sales, marketing and distribution rights for the product in the USA over the coming months. The iCast covered stent system will continue to be manufactured by Atrium Medical Corporation (Merrimack, USA), which is part of Getinge.
“This agreement with Cook Medical ensures that the iCast covered stent system will reach the optimum number of patients who will benefit from it in the USA,” said Patricia Fitch, president of Getinge in North America.
“This product fills the need of a covered stent in our vascular portfolio with a proven technology. iCast has five-year data aligned with our commitment to long-term clinical evidence and predictable results for [peripheral arterial disease] therapies,” said Mark Breedlove, senior vice president of Cook Medical’s vascular division.
Cook and Getinge continue to collaborate in other strategic areas, also partnering to complete the PRESERVE-Zenith branch endovascular graft-iliac bifurcation investigational device exemption (IDE) study with five-year follow-up. The purpose of this study was to evaluate the safety and effectiveness of the Zenith device in combination with the iCast covered stent in patients requiring treatment of aortoiliac and iliac aneurysms.