Royal Philips recently announced positive two-year results from the TOBA (Tack optimised balloon angioplasty) II below-the-knee (BTK) clinical trial.
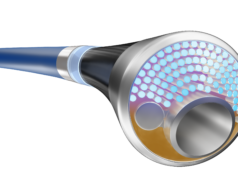
The data show the Philips Tack endovascular system (4F), a first-of-its kind dissection repair device, provides a sustained treatment effect and positive impact on quality of life for peripheral arterial disease (PAD) and chronic limb-threatening ischaemia (CLTI) patients at two years. George Adams (Rex Hospital, Chapel Hill, USA), co-principal investigator, presented the findings at the 2021 New Cardiovascular Horizons (NCVH) conference (1–4 June, New Orleans, USA).
Across all patients in the TOBA II BTK clinical trial at two years, 73.6% had freedom from clinically-driven target lesion revascularisation (CD-TLR) and did not require a repeat procedure for the treated artery segment. CD-TLR is a commonly used indicator of treatment efficacy durability. In the more complex CLTI patient population, which is typically associated with high rates of amputation and mortality, the data showed 94.7% target limb salvage (freedom from major amputation).
“The global endovascular community is diligently working to better understand how to restore blood flow in small limb vessels, promote healing and ultimately preserve limbs for people with CLTI, one of the most vulnerable and critical patient populations,” said Adams. “These positive two-year data reiterate the clinical importance of below-the-knee dissection repair and validates the sustained durability of Tack-optimised interventions.”
The two-year TOBA II BTK data also show sustained improvement in patients’ quality of life. In a patient questionnaire that assesses activity, pain, and overall health, patients report having more control of their health and increased improvement in mobility.
The Tack endovascular system is currently available for sale in the USA and some European Union countries. Further information, including safety information, is available here.