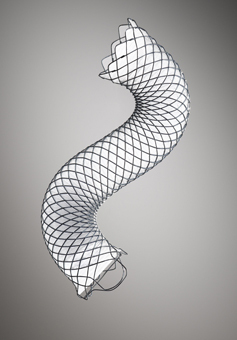
Boston Scientific has announced that the first patient has been enrolled in a study comparing its WallFlex Biliary RX Fully Covered self-expanding metal stent (SEMS) to plastic stents for the treatment of benign bile duct strictures caused by chronic pancreatitis.
This multicentre, prospective, randomised study will enrol 164 patients at leading hospitals in Australia, Austria, Belgium, Canada, France, Germany, Hong Kong, India, Italy and The Netherlands.
In a separate single-arm study, 187 patients were treated with the WallFlex Stent for multiple types of benign biliary strictures including, those caused by chronic pancreatitis. The stents implanted in that study were removed up to one year after being placed in the body. Five-year follow-up post stent removal is ongoing.
Preliminary data were presented at Digestive Disease Week 2012 by Jacques Deviere, Erasme Hospital, Brussels, Belgium. The data indicate that a SEMS can be removed safely any time up to one year post placement, and that short-term stricture resolution rates compare favourably with the results reported with plastic stents in chronic pancreatitis-related benign biliary strictures.
“Preliminary data show promising results for the treatment of chronic pancreatitis-related benign biliary strictures using SEMS, compared to literature on the use of plastic stenting,” said Puspok, Medical University of Vienna, Austria, an investigator in both studies who enrolled the first patient in the randomised study. “Multiple plastic stenting remains an established treatment choice for biliary strictures caused by chronic pancreatitis. However, this form of treatment requires multiple stent exchanges and the long-term success rate is low. Treatment with removable fully covered SEMS could overcome these limitations. A head-to-head comparison of both stenting treatment regimens is essential in order to collect robust data to guide physicians in the optimal treatment of their patients with chronic pancreatitis.”
SEMS, which have a significantly larger diameter than plastic biliary stents, have long been the standard of care for palliation of malignant biliary strictures. The studies above are evaluating the benefits of using a SEMS in benign biliary strictures, with an objective to demonstrate stricture resolution in fewer procedures.
“We are hopeful that the results of this study will demonstrate clinical benefit and cost effectiveness of a single metal stent approach versus plastic stenting, which typically requires multiple procedures,” said David Pierce president of the Endoscopy business at Boston Scientific.
About the WallFlex Biliary RX Fully Covered Stent
The WallFlex Biliary RX Fully Covered Stent has a silicone polymer Permalume Coating designed to reduce the potential for tumour/tissue ingrowth, and an integrated retrieval loop for removing or repositioning the stent in the event of incorrect placement during the initial procedure or for removal from benign strictures up to one year after placement. The stent is constructed of braided, platinum-cored Nitinol wire (Platinol Wire) and features three key components: radial force to help maintain duct patency and resist migration, flexibility to aid in conforming to tortuous anatomies and full-length radiopacity to enhance stent visibility under fluoroscopy.
The complete line of WallFlex stents‰Û¥fully covered, partially covered and uncovered‰Û¥is available in the United States and has CE mark approval for use in the palliative treatment of malignant biliary strictures. In addition, the WallFlex Biliary RX Fully Covered stent is CE-marked for the treatment of benign biliary strictures.
The WallFlex Stent is not approved in the United States for use in the treatment of benign biliary strictures. The safety and effectiveness of the stent for use in the vascular system have not been established.