
Lead author of the VAPOUR trial (Vertebroplasty for Acute Painful Osteoporotic fractURes) William Clark discusses the recent history of Australian Medicare funding for vertebroplasty, detailing how the 2009 results of two negative trials resulted in the Medicare Services Advisory Committee (MSAC) pulling their financial backing of the procedure. He outlines how the VAPOUR trial identified a subset of patients who benefit from the minimally invasive procedure, and ultimately led to the reinstatement of Medicare funding nationally.
Our application for public funding of vertebroplasty in Australia was based on the VAPOUR trial. Medicare funding for vertebroplasty had been removed after two negative trials were published together in 2009. We felt that these trials had largely excluded the group of patients that we had been treating in Sydney—patients with severe pain and early fractures, especially hospitalised patients. We conducted the VAPOUR trial to gather evidence in this patient group and the trial showed positive benefits from vertebroplasty.
Consistent with the recent history of vertebroplasty debate, the Medicare Services Advisory Committee (MSAC) assessment was shrouded in controversy. The MSAC was torn between the positive evidence of VAPOUR, which was specific for this application, and the negative findings of the Cochrane vertebroplasty review. The situation was complicated by the first author of the Cochrane review being a committee member of the MSAC. Timing of the Cochrane review appeared designed to influence the MSAC assessment.
MSAC initially advised against funding vertebroplasty, citing negative findings of the Cochrane review. The Interventional Radiology Society of Australasia (IRSA) complained to the Australian government that the application had not received an impartial hearing and that the Cochrane review should have been excluded due to conflict of interest. VAPOUR authors have published a criticism that the Cochrane review misrepresented evidence from our trial.1
The MSAC agreed to reconsider the application and summoned a stakeholder meeting for vertebroplasty in June 2019, inviting additional input from a broader range of clinical, epidemiologic, and biostatistical experts. The consensus from this meeting supported public funding of vertebroplasty for a subgroup of patients with uncontrolled pain from acute thoracolumbar vertebral fractures of not more than three-weeks duration. This patient subgroup derived most benefit from vertebroplasty in the VAPOUR trial. It includes most of the patients who are hospitalised with osteoporotic spinal fracture pain.
Early vertebroplasty can reduce pain and the reliance on opiate analgesia, with its attendant negative side-effects (delirium and constipation), which so often complicate management of these patients. It can change the natural history of the fracture by restoring height and preventing further collapse while the fracture is still soft and pliable.
Two randomised trials have assessed vertebroplasty for patients with fractures of three weeks or less. Diamond and colleagues2 published findings of a sub-group analysis from the VAPOUR trial, looking at 93 patients (46 vertebroplasty, 47 placebo) who underwent an intervention within three-weeks of fracture. Yang and colleagues3 published an open label randomised trial of 107 patients (56 vertebroplasty, 51 control) for vertebroplasty performed within three-weeks of fracture. Both trials showed significantly better pain reduction in the vertebroplasty group and worse complications in the control group.
The authors of the VAPOUR trial are proud that we have helped identify a patient group likely to benefit from vertebroplasty. We encourage interventional radiologists to consider offering early vertebroplasty for patients with severe pain or immobility caused by osteoporotic vertebral fractures. Delay can mean missing the window of opportunity to change the natural history of the fracture to benefit these suffering patients.
William Clark is an interventional radiologist at St George Private Hospital, Sydney, Australia.
References
- Clark W, Bird P, Diamond T, et al. The Cochrane vertebroplasty review misrepresented evidence for vertebroplasty with early intervention in severely affected patients. BMJ Evid Based Med. 2019
- Diamond, T et al. Early vertebroplasty within three weeks of fracture for acute painful vertebral osteoporotic fractures: subgroup analysis of the VAPOUR trial and review of the literature. Eur Spine J. 2020
- Yang EZ, Xu JG, Huang GZ, et al. Percutaneous vertebroplasty versus conservative treatment in aged patients with acute osteoporotic vertebral compression fractures. Spine 2016

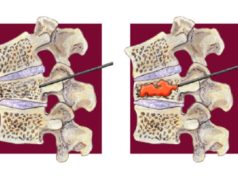








Well done for the persistence shown to give access to this technology for these patients.