The VAPOUR trial proves that vertebroplasty is effective in patients with severe pain and fractures of less than six weeks’ duration, when an adequate amount of cement is injected. Subgroup analysis suggests that benefits are maximal in thoracolumbar fractures, writes William Clark, Sydney, Australia.
There have been few more debated topics in medicine over the last decade than percutaneous vertebroplasty. Two masked, randomised trials1,2 published in 2009, showed vertebroplasty to be no more effective than a placebo. Two open label randomised trials of vertebral augmentation, the FREE trial3 of kyphoplasty and VERTOS II4 trial of vertebroplasty, showed efficacy for both techniques compared to usual care. Because masked trials constitute a higher level of evidence than open label trials, it was necessary to prove the efficacy of vertebroplasty within a masked trial format.
The VAPOUR trial5 (Vertebroplasty for acute painful osteoporotic fractures) used the same trial methodology as the previous masked trials but with different patient selection and procedural technique. Selection criteria were Numeric Rated Scale (NRS) pain ≥7/10, pain duration <six weeks, MRI evidence of recent fracture, and both hospital inpatients and outpatients included. This was very different to previous masked trials which were exclusively outpatient trials, with pain >3/10, with the duration of pain up to 12 months. We targeted patients with the most painful acute fractures of less than six weeks duration.
The trial team included four centres in Sydney, Australia, an independent data collection agency and independent statisticians. Data was collected over a six-month period. Patients, data collectors, and data remained masked until trial completion. Independent statisticians then unmasked and analysed the data. This is a robust trial framework at low risk of bias.
Randomisation was provided by the National Health and Medical Research Council by automated telephone service once the patient was in the procedure room. All patients received intravenous midazolam and fentanyl before the procedure. Vertebroplasty was performed with AVAmax kits (CareFusion, whose vertebral augmentation portfolio was bought by Stryker earlier this year) using a “vertebral fill approach”, distributing polymethyl methacrylate (PMMA) cement from the top to bottom, side to side and from anterior cortex to posterior third of vertebral body. The treating radiologist had no other role in the trial after the procedure. Placebo intervention simulated a vertebroplasty with skin incision, subcutaneous lidocaine and verbal and tactile cues suggesting that a vertebroplasty was being performed.
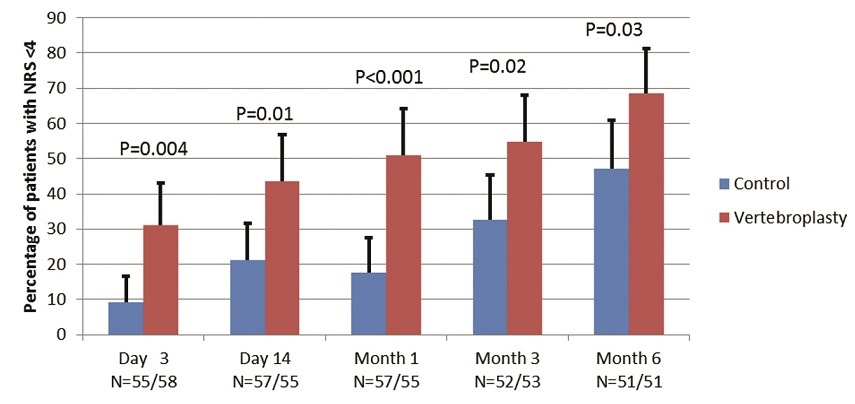
Between November 2011 and December 2014, we enrolled 120 patients and all underwent the treatment they were randomised to. Mean cement volume was 7.5cc (compared to 2.7cc in previous sham trials). The primary endpoint was the proportion of patients with low NRS pain scores (<4/10) at 14 days. A significantly greater proportion of patients in the vertebroplasty group achieved the primary outcome compared with the control group (44% vs 21%; between-group difference 23 percentage points, 95% CI 6–39; p=0.011). The advantage in this outcome for vertebroplasty remained similar at every data collection time point to six months, with a maximum between-group difference at four weeks of 33% (95% CI, 17 to 50) (Figure 1).
There were also positive secondary outcomes in the vertebroplasty group. Mean NRS pain score was lower at every time point, Roland Morris Disability score was lower from one month to six months, analgesic use was lower at three months and six months, median duration of hospitalisation was lower by 5.5 days, and fractured vertebral body height was 36% greater than placebo at six months. The reduction in hospital stay shows the procedure to be cost effective.
There were two serious adverse events in the vertebroplasty group. The first was hypoventilation following intravenous sedation, before the procedure commenced, and this was reversed and the procedure re-scheduled two days later. The second was a humerus fracture sustained during transfer into the prone position, and this was treated with plaster cast successfully. Two patients in the placebo group developed spinal cord compression due to continued vertebral collapse and fracture retropulsion in the weeks following randomisation. One had successful spinal decompressive surgery and the other became paraplegic. Neither had significant retropulsion at the time of enrolment.
Subgroup analysis of the primary endpoint was performed according to spinal segments—thoracic (T5–T10), thoracolumbar (T11–L2), and lumbar (L3–L5). Logistic regression analysis showed significantly greater benefit in the thoracolumbar segment than in the other segments (p=0.001).
In conclusion, the VAPOUR trial proves that vertebroplasty is effective in patients with severe pain and fractures of less than six weeks’ duration if adequate amount of PMMA is injected. Subgroup analysis suggests that benefits are maximal in thoracolumbar fractures.
The study was funded by an unrestricted research grant from CareFusion Corporation, who had no role in trial design, conduct, analysis or publication.
William Clark is director, Interventional Radiology, St George Private Hospital, Sydney, Australia. Clark has reported no disclosures pertaining to this article.
References
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361: 569–79
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361: 557–68.
- Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009; 373: 1016-24.
- Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet 2010; 376: 1085-92
- Clark W, Bird P, Gonski P, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2016; published online 17 August.