Nearly 10 years have passed since Aravind Arepally et al published data from the first gastric artery chemoembolization preclinical trials for the treatment of obesity. Since that time, preclinical trials with bland embolic microspheres rather than chemoembolization have shown the most promise in terms of safety and efficacy. This once radical obesity treatment idea (bariatric artery embolization) is now becoming more widely accepted as early human clinical trials at several sites are showing promising preliminary results.
The mechanism through which bariatric artery embolization is thought to trigger weight loss is through modulation of the ghrelin hormone. Ghrelin is a 28-amino-acid peptide hormone produced and secreted predominately by mucosal X/A cells lining the gastric fundus in humans. Ghrelin is thought to be the principal appetite-inducing stimulus in humans and has an influential role on caloric energy balance. Following embolization, decreased serum ghrelin levels and suppression of appetite resulting from ischaemia may be responsible for the weight loss observed in both preclinical and early human trials.
The history of transarterial obesity treatments explored in preclinial trials includes some caustic agents: belomycin A5 hydrocloride and sodium morrhuate have both been used as chemoembolics, while calibrated microspheres down to 40μm in size have been used as bland embolics. However, in spite of the preclinical success of some of these agents, the use of yttrium-90 (Y-90) radioembolization in a procedure similar to bariatric artery embolization might initially seem contraindicated. This is largely due to the perceived hypersensitivity of the gastrointestinal tract to radiation—a fallacy commonly promoted among interventional radiologists. In fact, data from external-beam radiation therapy tells us that the gastrointestinal tract is probably less radiosensitive than normal liver tissue, where Y-90 radioembolization is most commonly used as a treatment for liver cancer.
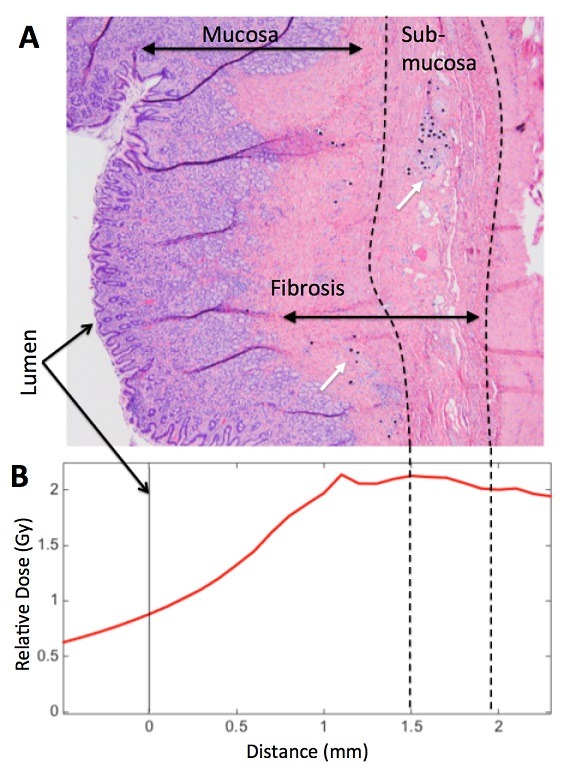
While a potential complication in radioembolization for treatment of liver cancer is gastrointestinal ulceration following non-target embolization of hepatoenteric collateral vessels, both bland and chemoembolization can also result in such ulceration.
A recent paper from our group in the Journal of Vascular and Interventional Radiology has described a bariatric arterial radioembolization procedure using Y-90 microspheres in a preclinical model. While the goal of bariatric arterial embolization is to use ischaemia to initiate a change in orexigenic hormone levels and energy balance, using radioembolization is associated with minimal tissue ischaemia. Instead, it uses a localised radiation burden to affect ghrelin hormone production.
Further, preclinical data suggested that weight loss may also be achieved through a restrictive component to this therapy resulting in decreased stomach size and volume. Both a strength and a weakness of Y-90 bariatric radioembolization therapy compared to ischaemic bariatric artery embolization is that the former may allow for more precise targeting of ghrelin-producing cells. Immunohistochemistry of the gastric fundus has shown that the greatest concentration of ghrelin producing cells is deep in the fundal mucosa. The goal of bariatric arterial radioembolization studies was to eliminate as many ghrelin-producing cells as possible, while sparing superficial gastric mucosa to avoid potential ulceration and side effects. This was accomplished through pretreatment planning using an injectable radiopharmaceutical as a Y-90 microsphere surrogate, similar to established techniques in hepatic radioembolization. Careful dosimetry based on pretreatment planning combined with suitable sizing of the microsphere allowed for deposition in the sub-mucosa for complete ablation of the deep mucosa, including ghrelin-producing cells. At the same time, a rapid fall off in radiation dose ensured that superficial mucosal integrity was maintained.
Of course, while this planning process led to an elegant targeted solution, the technical complexity greatly exceeds that of conventional bariatric arterial embolization. Critics of Y-90 bariatric artery embolization have pointed out potential radiation risks associated with this use of radiation therapy in the treatment of benign disease. However, morbid obesity can hardly be called a benign disease. Like bariatric artery embolization, bariatric artery radioembolization is not a potential intervention for patients wishing to lose a few pounds to look better in their swimsuit. It is a potential therapy for morbidly obese individuals that are refractory to diet and exercise and for whom bariatric surgery is the only remaining intervention.
While radiation is associated with increased probability of future neoplasia, this risk is substantially less than the 30-day mortality associated with traditional surgical obesity intervention, which can exceed 1%. Where this therapy will fit among the numerous transarterial approaches that have been attempted in animal models for the treatment of obesity is uncertain. However, even if this therapy is never attempted in a human, it strengthens the argument that left gastric artery embolization with a variety of therapeutic embolic agents is a realistic and potentially safe treatment modality for morbid obesity. Initial investigations into Y-90 bariatric artery radioembolization also have had important implications regarding the use of radioembolization in the treatment of liver cancer. We now have a much better understanding of the microscopic changes that occur in gastrointestinal tissue following radioembolization.
In particular, Y-90 radioembolization may not be as toxic to the gastrointestinal tract as was once thought as we have recently described in the Journal of Nuclear Medicine. While gastrointestinal damage may occur with substantial Y-90 non-target embolization, excessive prophylaxis is probably unnecessary since small doses are not likely to result in clinical sequelae.
Alexander S Pasciak is a clinical associate professor of Radiology at the University of Tennessee Graduate School of Medicine, Knoxville, USA. He received grant funding from Sirtex in support of a preclinical study in the use of SIR-Spheres for the treatment of obesity.