Gilles Soulez, Montréal, Canada, delivered the Andreas Gruentzig lecture at the Cardiovascular and Interventional Radiological Society of Europe’s annual meeting (26–30 September, Lisbon, Portugal). “We need to change the way we look at abdominal aortic aneurysms,” he urged.
“We are currently just looking at the size of abdominal aortic aneurysm and how we can fit a stent-graft into it with little consideration for the underlying condition and the mechanisms associated with endovascular aneurysm repair (EVAR) success, or failure,” Soulez, professor of Radiology University of Montreal and CHUM Research Center, told delegates at the conference. He then outlined approaches to address the difficulties in visualisation of the area needing treatment and noted that advanced image modelling of abdominal aortic aneurysm could impact EVAR management.
“EVAR has the brilliant concept of endovascular bypass and it was developed by Juan Parodi and others in the early 1990s. The working hypothesis is that endoluminal exclusion of the diseased portion of the aorta will lead to sac thrombosis and healing of the abdominal aortic aneurysm,” said Soulez.
He also pointed out that the shortcomings of the hypothesis are that the success of the approach is dependent on the anatomy (feasibility ranges between 50 and 70%) and that its success and durability rely on the quality of the landing zones and the device used. Further, there is no ligation of collateral vessels, which could result in type II endoleak.
For 25 years, Soulez has been involved in developing medical imaging technologies to prevent complications that can occur with the treatment and monitoring of patients with abdominal aortic aneurysms. The main problem has been the ability to properly visualise the area to be treated.
“Remarkable advances in imaging have improved surgery and helped to develop less invasive interventions. But the images are still far from perfect. We want to develop new software to maximise the use of images generated with current ultrasound, scanning, and magnetic resonance imaging (MRI) technologies to ultimately provide more personalised treatments,” he explained.
Noting that there was room for improvement with EVAR, Soulez proceeded to outline the main clinical and imaging challenges with the procedure.
“First of all we need to improve rupture prediction with new imaging biomarkers (ie, we need to go beyond maximal diameter), we need to improve EVAR planning to minimise the complications [of the procedure] such as endoleak, endotension, limb occlusion and we need to integrate biomechanical simulation. [Further] we need to improve EVAR guidance, minimise procedure time and radiation exposure and contrast induced nephropathy and we also need to improve EVAR follow-up in terms of costs, radiation and contrast-induced nephropathy and monitor the healing process of aneurysms.”
Currently, a simple abdominal ultrasound measurement of the aorta can detect patients at risk of aneurysm rupture. Beyond 5cm for women and 5.5cm for men, and accelerated growth (defined as greater than 10mm in one year) intervention is usually recommended. However, researchers want to refine screening to provide the most appropriate treatments for patients who really need intervention.
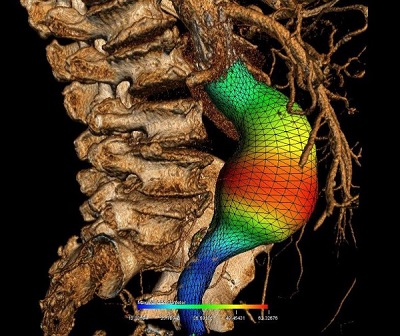
Soulez’s research provides three-dimensional images of all components of the aneurysm, ie. the light, the thrombus or clot, the wall, and the calcification. “The grid is used to establish growth profiles of the aneurysm. We are now working to create simulations to better predict the risk of rupture, adding biomechanical properties such as tissue elasticity and connectivity at each pixel of the grid,” he explained.
After EVAR imaging surveillance relies on CT-scanning and Doppler ultrasound. Ultrasound combined (or not) with contrast is more used to decrease the cost, and the hazard related to irradiation and contrast induced nephropathy. Soulez, in collaboration with Guy Cloutier, also of the University of Montreal Hospital Research Centre, is evaluating if ultrasound elastography can distinguish between the immature thrombus as seen with growing aneurysms (that is associated with EVAR failure) and the dense fibrous aggregation of the thrombus, that is associated with successful healing (pointing to the success of the procedure). This technology seems also to be sensitive to detect low flow endoleaks that are difficult to detect on CT-scanner.
Virtual reality
Simulations will also help in the operating room. Currently, EVAR is performed using static images taken by a scanner before the procedure. The procedure itself is done under fluoroscopy. “The image produced by X-ray shows the dye in the vessels and the stent being inserted, but not the wall. This approach requires a lot of dye, which can be toxic for the patient if used in excessive amounts,” said the radiologist.
Soulez’s laboratory has been able to develop an approach that combines all the available data. “We superimpose the images, and this helps to visualise the area to be treated. But in reality, the tools we introduce into the body during the procedure deform the organs. We are testing a new approach that uses a computer to automatically recognise the tools introduced into the body and correct the deformities they cause,” he said. “We hope this simulation-operation model will improve the accuracy of the procedure.”
These technologies could be particularly relevant for advanced EVAR cases such as fenestrated, branched or chimney procedures or EVAR in challenging necks that are at risk of developing endoleak and late failure. During the Andreas Gruentzig lecture at CIRSE, Soulez noted that future developments would include the use of stereotactic fluoroscopy shots instead of C-arm CT to perform 2D or 3D registration. There was also a need for automated workflow and the inclusion of simple biomechanical assumptions to optimise for vessel deformation.
“I hope that I have brought you several arguments to convince you that imaging and mechanobiology will improve EVAR management. The assessment of abdominal aortic aneurysm wall biomechanics should improve patient selection before intervention. I believe that biomechanical simulation and 3D roadmap will minimise complications in complex EVAR procedures because we are extending the indications very far,” Soulez said.
“There is a growing evidence that ultrasound will play a key role in patient surveillance and less invasive CT surveillance for EVAR follow-up [will be important]. The imaging of sac healing will be important to evaluate new therapies, such as the long-term results of endovascular aneurysm sealing (EVAS), bioactive stents or injection of a gel around the implant to prevent or stop leaks as well as stem cell therapy,” he said.