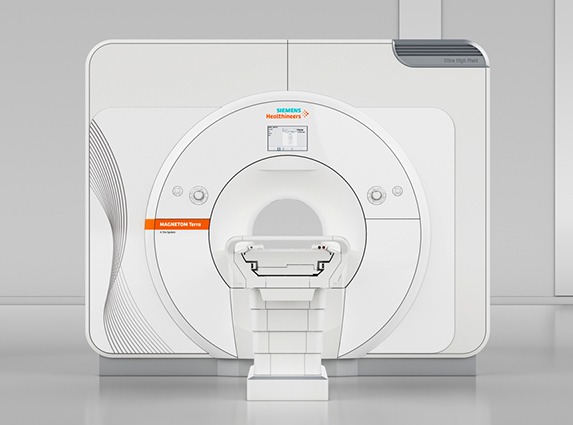
The US Food and Drug Administration has cleared the first seven tesla (7T) magnetic resonance imaging (MRI) device, more than doubling the static magnetic field strength available for use in the USA. The Magnetom Terra (from Siemens) is the first 7T MRI system cleared for clinical use in the USA.
“The overall image quality of MRI improves with higher magnetic field strength,” stated Robert Ochs, director of the Division of Radiological Health in the FDA’s Center for Devices and Radiological Health. “The added field strength allows for better visualisation of smaller structures and subtle pathologies that may improve disease diagnosis.”
Before this clearance by the FDA, clinical MRI systems in the USA were available in field strengths of 3T and below.
According to a release from the FDA, it reviewed the Magnetom Terra through the 510(k) premarket clearance pathway. The FDA based its clearance on comparison to a predicate device and acquisition of sample clinical images. The agency reviewed the safety of the radiofrequency subsystem through computational modelling, simulations and rigorous experimental validation.
The manufacturer also provided data from a comparative study of 35 healthy patients that compared images of the patients using the 7T device and images using the 3T device. Board-certified radiologists reviewed the images and confirmed that the images acquired on the 7T device were of diagnostic quality and, in some cases, an improvement over imaging at the 3T.
The Magnetom Terra is for patients who weigh more than 66 pounds, and is limited to examinations of the head, arms and legs (extremities).