![]()
 The REDUCED-1 study, a US Food and Drug Administration (FDA) investigational device exemption (IDE)-approved pilot study of the treatment of hypertension with the proprietary Therapeutic Intra-Vascular Ultrasound (TIVUS) system (SoniVie), has completed enrolment.
The REDUCED-1 study, a US Food and Drug Administration (FDA) investigational device exemption (IDE)-approved pilot study of the treatment of hypertension with the proprietary Therapeutic Intra-Vascular Ultrasound (TIVUS) system (SoniVie), has completed enrolment.
“Initial results from the ongoing REDUCED-1 study are encouraging and we continue to diligently follow the enrolled patients,” said Christian Spaulding, chief medical officer of SoniVie, in a press release announcing the study milestone.
The trial had two enrolment cohorts that were conducted under an identical protocol in the USA and in Israel. Twenty-five patients were enrolled in the USA and 15 patients were enrolled in Israel. All patients (N=40) are now in the follow-up phase of the study where primary efficacy (change in daytime systolic ambulatory blood pressure) will be analysed at three months and safety will be analysed at one month and 12 months follow-up respectively.
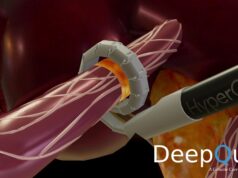
Renal Denervation with TIVUS is a minimally invasive procedure that uses high-frequency non-focused ultrasound energy to ablate nerves in the renal arteries. This causes a reduction in the nerve activity, which may decrease blood pressure. This procedure is designed for patients who suffer from resistant hypertension.
“Our next commitment towards patients, physicians and regulators is now to clinically validate the TIVUS system in a global pivotal trial and expand its use under the pivotal study with radial access procedures,” said Tomaso Zambelli, chief executive officer, SoniVie.