Thrombolex recently announced the results of the RESCUE trial’s prespecified interim analysis, of the first 62 evaluable patients, in a late-breaking clinical trials session at Vascular Interventional Advances (VIVA) 2021 (5–7 October, Las Vegas, USA).
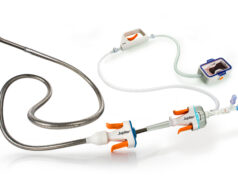
This PIVOTAL trial is scheduled to enrol at least 100 evaluable patients with acute intermediate-risk pulmonary embolism. This trial is evaluating the efficacy and safety of the Bashir and Bashir S-B endovascular catheters (BECs) in the treatment of acute PE under an investigational device exemption (IDE) from the US Food and Drug Administration (FDA). The Bashir endovascular catheter is intended for the controlled and selective infusion of physician-specified fluids, including thrombolytics, into the peripheral vasculature. It is also now intended for the controlled and selective infusion of physician-specified fluids, including thrombolytics, into the peripheral vasculature, enabling the restoration of blood flow in patients with venous thrombus.
The goal of RESCUE is to achieve an additional indication for use of these devices in the treatment of acute pulmonary embolism.
This prespecified interim analysis was presented by Akhilesh Sista, chief of Interventional Radiology, NYU Langone Health, New York, USA. The analysis included 44 males and 18 females with an average age of 58.5 years; 90.3% had high intermediate-risk pulmonary embolism with both right ventricular dysfunction and elevated biomarkers. Each patient was treated with 7mgs of r-tPA into each pulmonary artery over five hours (a total of 14 mg r-tPA in 58 patients with bilateral pulmonary embolism and 7mg in four patients with unilateral pulmonary embolism) at 18 participating centres in the USA.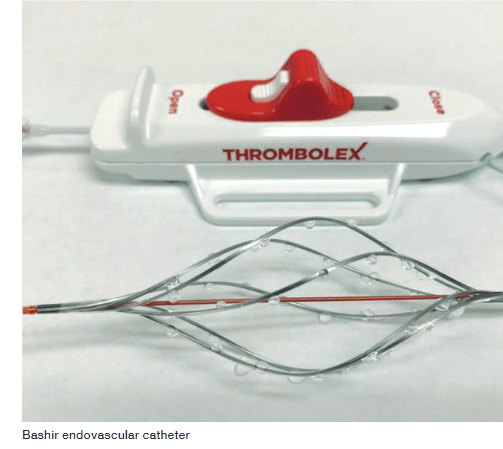
Computed tomography (CT) scan at 48 hours after infusion showed that the right ventricular to left ventricular (RV/LV) diameter ratio decreased by 32.1% (0.52±0.38: 95% confidence interval [CI] 0.42–0.62, p<0.0001), and the pulmonary clot burden by Refined Modified Miller Index had decreased by 36.3% (8.4±4.3: 95% CI 7.34–9.5, p<0.0001). The successful device placement rate was 100%, there were no device-related complications and the major bleed rate at 72 hours was zero.
“These interim results from the RESCUE trial are very exciting,” said Sista. “The most notable finding is the core lab-assessed reduction in pulmonary thrombus burden at 48 hours, which may translate to better short- and long-term outcomes pending further research. The reduction in RV/LV ratio and the absence of device- or drug-related adverse events are also salutary outcomes. The expandable infusion basket of the device, with the pharmacomechanical mode of action, appears to restore blood flow promptly. I look forward to the final results of RESCUE and subsequent investigations that will determine the role of this promising novel technology in the treatment of acute pulmonary embolism.” Brian Firth, chief scientific officer of Thrombolex and principal investigator on the Small Business Innovation Research grant from the National Heart Lung and Blood Institute (NHLBI) stated: “The strong support and encouragement that we have received from the NHLBI as we have conducted this trial, especially in the midst of the COVID-19 epidemic, has really made this trial possible.”