Tag: ReFlow Medical
US FDA grants de novo clearance for Reflow Medical’s Spur peripheral...
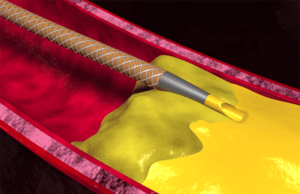
Reflow Medical recently announced that the US Food and Drug Administration (FDA) has granted de novo clearance for the company’s Spur peripheral retrievable stent...
Reflow Medical completes enrolment in the DEEPER REVEAL clinical trial
Reflow Medical has announced completion of enrolment in the DEEPER REVEAL clinical trial to evaluate the Reflow Spur stent.
The company notes in a...
Reflow Medical receives CE mark for Bare Temporary Spur stent system
Reflow Medical recently announced it has received CE mark certification in the European Union for the Bare Temporary Spur stent system. The device is...
Reflow Medical introduces Spex LP shapeable reinforced support catheter
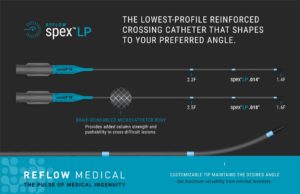
Reflow Medical introduces the low-profile Spex LP 0.014 and 0.018-inch reinforced support catheters.
According to the company, the Spex LP is designed with a low-profile...
Reflow Medical receives approval in Japan for the Wingman catheter
Reflow Medical has announced that Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) has approved the Wingman chronic total occlusion (CTO) catheter. Reflow Medical has...
Reflow Medical wins FDA breakthrough nod for Temporary Spur stent system
Reflow Medical has announced that its Temporary Spur stent system for treating below-the-knee peripheral arterial disease (PAD) won US Food and Drug Administration (FDA)...
VIVA 2019: Temporary Spur stent system appears to be feasible and...
Today at the 2019 Vascular Interventional Advances conference (VIVA; 4–7 November, Las Vegas, USA), Thomas Zeller (Bad Krozingen, Germany) gave an update on the...
Reflow Medical completes enrolment in the Wing-IT IDE CTO clinical trial
Reflow Medical has announced the completion of enrolment in its Wing-IT investigational device exemption (IDE) trial; a prospective, multicentre, nonrandomised study evaluating the ability...