Tag: Gore
Poll highlights rising concern for radiation exposure among interventionists
A new US-wide poll announced today by Gore and Egg Medical sheds light on the growing concern for radiation exposure in operating rooms and...
Gore announces US FDA approval of expanded Viatorr TIPS endoprosthesis
Gore has announced US Food and Drug Administration (FDA) approval of a new 6–10mm diameter range for the Viatorr transjugular intrahepatic portosystemic shunt (TIPS)...
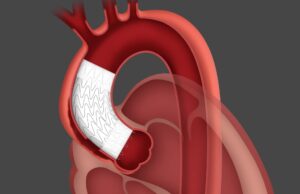
Gore Tag thoracic branch endoprosthesis receives expanded FDA approval for endovascular...
Gore has announced that the Gore Tag thoracic branch endoprosthesis (TBE) is now approved by the US Food and Drug Administration (FDA) for use...
Gore receives CE mark for lower profile Viabahn VBX endoprosthesis
Gore has announced recent CE mark of a lower profile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft).
Medical Device Regulation (MDR) approval of this...
Gore’s Viabahn VBX stent graft receives FDA approval
Gore has announced recent US Food and Drug Administration (FDA) approval of a lower profile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft).
"Our team...
First patients enrolled in Gore’s VBX FORWARD clinical study
Gore has announced that the first patients have been enrolled in the Gore VBX FORWARD clinical study, a global prospective, multicentre, randomised controlled trial...
First patient treated in ARISE II study of Gore ascending stent...
Gore has announced the first patient implantation of the Gore ascending stent graft in the ARISE II trial, describing this as an exciting step...
Medtronic announces first enrolment in head-to-head randomised trail evaluating durability of...
Medtronic has announced the first patient enrolment in the ADVANCE Trial, a head-to-head randomised controlled trial of two leading aortic stent graft systems, the...
Gore acquires InnAVasc Medical
Gore has announced the acquisition of InnAVasc Medical, a privately held medical technology company focused on advancing care for patients with end-stage renal disease...
Gore enhances Viabahn endoprosthesis portfolio with lower profile delivery
Gore has announced the US launch of the lower profile, large diameter Gore Viabahn endoprosthesis.
Gore previously received approval from the US Food and Drug...
Gore Tigris vascular stent demonstrates high patency rates at 12 months
New results suggest that the Tigris vascular stent (Gore) is a safe and effective device that can be incorporated into a modern “leave-nothing-behind” treatment...
Gore moulding and occlusion balloon for endovascular aortic repair receives approval...
Gore has announced FDA 510(k) clearance, approval from the Japanese Ministry of Health, Labour, and Welfare, and receipt of CE mark for the innovative...
First patient enrolled in investigational study of Gore Excluder conformable endoprosthesis...
W L Gore and Associates has announced the first implant of the Gore Excluder conformable abdominal aortic aneurysm endoprosthesis in the USA. The procedure...
First implant placed in Gore Tag thoracic branch endoprosthesis pivotal study
Gore has announced the placement of the first implant of the Gore Tag thoracic branch endoprosthesis in the pivotal study. The patient was enrolled...
Gore Tigris vascular stent gains FDA approval for treatment of peripheral...
Gore has announced US Food and Drug Administration (FDA) approval of the Gore Tigris vascular stent, a dual-component stent with a unique fluoropolymer/nitinol design....