Tag: DETOUR
Endologix initiates postmarket study of the Detour system
Endologix has announced the initiation of the Percutaneous transmural arterial bypass (PTAB)1 postmarket study. This study marks the beginning of a comprehensive postmarket study...
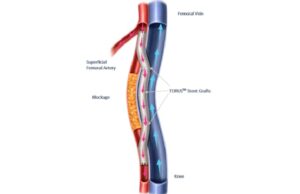
Endologix announces completion of enrolment in TORUS 2 study for PAD...
Endologix has completed enrolment in the TORUS 2 investigational device exemption (IDE) clinical study in the USA, a press release reports.
The TORUS 2...
DETOUR I two-year outcomes: “Excellent” functional improvement in complex PAD cohort
During a late-breaking data session at this year’s Vascular Interventional Advances annual meeting (VIVA 2020; 6–8 November, virtual), Ehrin Armstrong (University of Colorado, Denver,...
PQ Bypass receives IDE approval to initiate pivotal DETOUR II clinical...
PQ Bypass has received conditional approval of its investigational device exemption (IDE) from the US Food and Drug Administration (FDA) to initiate the pivotal...