Tag: AVF
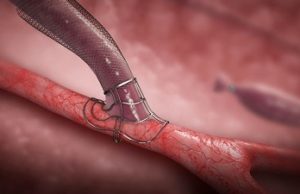
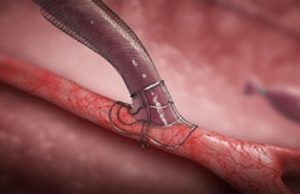
AVeNEW trial shows superior AVF target lesion patency with covered stent...
“Study design is critically important when interpreting outcomes,” said Bart Dolmatch (Mountain View Hospital, Mountain View, USA) in his evaluation of the “disconnect” between...
Laminate Medical announces first implantation of VasQ AVF device
Laminate Medical has announced the first implantation of their VasQ device in the USA, performed by Ari Kramer (Spartanburg Regional Hospital, Spartanburg, USA). The...
VOLA II trial results to ‘change practice’ and ‘improve outcomes’
“We wanted to find a common language between interventional radiologists and nephrologists and we wanted to be sure that the angioplasty we have done...
Laminate Medical announces FDA clearance for VasQ AVF creation device
Laminate Medical Technologies has announced their flagship device, the VasQ External Vascular Support, has been cleared by the US Food and Drug Administration (FDA)...
Multi-society guidelines on varicose vein management published
The Society for Vascular Surgery (SVS), American Venous Forum (AVF), and American Vein and Lymphatic Society (AVLS) have released the second and final part...
Enrolment completed in SAVE trial of Selution SLR for AVF treatment
MedAlliance has announced completion of patient enrolment in the SAVE clinical trial with the Selution Sustained Limus Release (SLR) 018 drug-eluting balloon (DEB) for...
WRAPSODY Arteriovenous Access Efficacy (WAVE) pivotal study completes enrolment
Merit Medical has announced that is has completed enrolment in its WRAPSODY Arteriovenous Access Efficacy (WAVE) pivotal study. Merit’s WAVE study is a prospective,...
Alucent Biomedical granted US FDA approval for clinical study
Alucent Biomedical has announced that the US Food and Drug Administration (FDA) granted an investigational device exemption (IDE) for a US clinical study of...
VentureMed completes enrolment of Flex Vessel Prep system randomised controlled trial...
VentureMed Group recently announced that it has completed enrolment of a randomised controlled trial (RCT) titled 'Flex Vessel Prep prior to PTA for the...
Largest real-world experience to date with VasQ device indicates long-term benefits...
The VasQ external support device (Laminate Medical) has demonstrated long-term benefits for radiocephalic (forearm) arteriovenous fistula (AVF) creation in a retrospective analysis of 150...
AVF 2022: Six-month CLOUT data indicate ClotTriever can effectively remove full...
Six-month outcomes from the ongoing CLOUT registry demonstrate the “safety and efficacy” of the ClotTriever thrombectomy system (Inari Medical) in a real-world deep vein...
AVF 2022: Emerging autogenous venous valve formation system sees “continual improvement”
An emerging endovenous valve formation system designed to treat patients with chronic venous insufficiency (CVI) with evidence of deep venous reflux has demonstrated continual...
IMPRESSION trial assessing MagicTouch AVF passes 50% enrolment
Concept Medical has shared the latest update from its IMPRESSION (Sirolimus-coated balloon angioplasty versus plain balloon angioplasty in the treatment of dialysis access dysfunction)...
Arrow recalls percutaneous thrombolytic device kits over risk of separation
Arrow International has recalled its Arrow-Trerotola over-the-wire 7Fr percutaneous thrombolytic device (PTD) kits, which are used to remove clots in adult patients who have...
Benefits of percutaneous fistula creation further bolstered by five-year Ellipsys data
The Ellipsys vascular access system (Avenu Medical/Medtronic) can be used to easily and safely create durable percutaneous arteriovenous fistulas (pAVFs) for haemodialysis—with new, long-term...
“Very encouraging” 12-month data from WRAPSODY FIRST study presented at CIRSE...
Twelve-month results from a first-in-human study of the Wrapsody cell-impermeable endoprosthesis (Merit Medical Systems) for the treatment of access circuit stenosis in haemodialysis patients...
Results from first-in-human study of Amplifi vein dilation system presented at...
Preliminary clinical results from a first-in-human study assessing the Amplifi vein dilation system (Artio Medical) were presented at Vascular Interventional Advances (VIVA) 2021 (5–7 October...
Artio Medical completes enrolment of first-in-human trial investigating Amplifi vein dilation...
Artio Medical announced today it has completed enrolment in its first-in-human study evaluating the Amplifi vein dilation system. In the study, five patients were treated...
Arteriovenous fistulas contribute to higher survival of haemodialysis patients with COVID-19
A new study, published online in The Journal of Vascular Access (JVA), suggests that arteriovenous fistulas (AVFs) contribute to higher survival of haemodialysis patients...
Vascular Therapies announces clinical results from phase 3 randomised multicentre clinical...
Vascular Therapies recently announced results from its phase 3 clinical trial in which Sirogen showed encouraging arteriovenous fistula (AVF) outcomes in elderly end-stage renal...
Artio Medical announces successful first human use of the Amplifi vein...
Artio Medical recently announced that it has successfully completed the first human use of its Amplifi vein dilation system. The first clinical procedure was...
Arterial diameter may help to predict AVF aneurysm progression, study suggests
A recent study concludes that arterial diameter may influence arteriovenous fistula (AVF) aneurysm progression and the interval to surgical revision. “Patients with larger arterial...
VasQ device improves AVF creation in haemodialysis patients, JVA study reports
VasQ, a high haemocompatibility biosynthetic vascular device from Laminate Medical Technologies, could be protective against the haemodynamic modifications that occur during arteriovenous fistulae (AVF)...
Patient-specific computation model may improve AVF maturation rates
The results of a randomised controlled trial—the Shunt simulation study—show that a new patient-specific computation model accurately calculated postoperative access flow. As flow is...
Artio Medical acquires Flow Forward Medical, expanding peripheral vascular portfolio
Artio Medical today announced it has acquired Flow Forward Medical, a medical device company developing methods for establishing and maintaining vascular access sites.
This...
APERTO becomes the first drug-coated balloon to be approved in China...
APERTO, Cardionovum’s paclitaxel-coated balloon for arterio-venous access, has received market approval for China, making it the first high pressure drug-coated balloon (DCB) available in...
BIBA Briefings: FDA approval of IN.PACT AV is a boon for...
Last month (November), the FDA approved Medtronic’s paclitaxel-coated balloon IN.PACT AV for the management of failing arteriovenous fistulae (AVF). Approval for the device was based...
C-TRACT trial on image-guided procedures for mitigating post-thrombotic syndrome partners with...
Writing to members of the American Venous Forum (AVF), president Brajesh K Lal (Baltimore, USA), has revealed that the AVF has established a partnership...
Comparing and contrasting surgically-created AVFs with those made by endovascular means
Nicholas Inston (Birmingham, UK) tells Vascular News at LINC 2019 about the exciting field of percutaneous arteriovenous fistula (AVF) creation, the main devices and...
Latest data on Lutonix and IN.PACT drug-coated balloons in AV access...
There in an increasing physician interest in exploring the role of drug-coated balloons in arteriovenous (AV) access. At the Leipzig Interventional Course (LINC; 30...
Meta-analysis shows positive experience for patients who received an endovascular arteriovenous...
An international meta-analysis of clinical experience in patients who received an endovascular arteriovenous fistula (endoAVF) for haemodialysis access was presented at Leipzig Interventional Course...
Twelve-month NEAT trial data promising for everlinQ endoAVF haemodialysis access system
TVA Medical has announced the online publication of positive results from the Novel Endovascular Access Trial (NEAT) evaluating its everlinQ endoAVF system. The 12-month...
Lutonix AV results provide tailwind for drug-coated balloons in dysfunctional arteriovenous...
Scott Trerotola presented the first release of eight-month data from the Lutonix AV IDE trial at the Leipzig Interventional Course (LINC; 24–27 January, Leipzig,...