Medtronic has announced it received approval in Japan from the Pharmaceuticals and Medical Devices Agency (PMDA) for its Symplicity Spyral renal denervation system for the treatment of resistant hypertension. The company will now initiate the process to obtain approval for insurance coverage.
In Japan, hypertension is a significant health concern, affecting approximately 43 million adults. Despite widespread awareness, only about 60% of those diagnosed with high blood pressure receive treatment, and just under half of those treated achieve adequate blood pressure control, according to figures published in Nature.
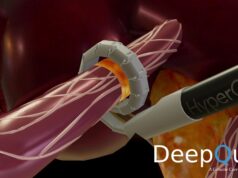
Symplicity uses a single thin tube to deliver radiofrequency energy to calm the nerves near the kidneys that can become overactive and cause elevated blood pressure.
“As hypertension continues to pose a growing health challenge in Japan, the demand for advanced and alternative treatment options is becoming increasingly critical, especially for Japan’s ageing population,” stated Jason Weidman, senior vice president and president of the Coronary and Renal Denervation business within the Cardiovascular Portfolio at Medtronic. “This year marks 50 years of Medtronic serving patients in Japan. The recent approval by the PMDA underscores our dedication to advancing healthcare innovation and delivering transformative therapies to those in need. We are excited to continue our momentum and leadership in the renal denervation space by expanding patient and physician access to the Symplicity blood pressure procedure.”
Medtronic clinical trials, including the SPYRAL HTN-ON MED study, showed 24-month office systolic blood pressure reduction of 17.4mmHg (Spyral patients) through two years and significantly greater reduction versus sham. The SPYRAL HTN clinical programme has shown long-term reductions without the need for additional medication.
The Medtronic SPYRAL HTN global clinical programme has studied renal denervation in more than 5,000 patients in the presence and absence of medication, and with high baseline cardiovascular risk, and is backed by experience in over 30,000 patients globally.
The Symplicity Spyral renal denervation system is approved for commercial use in over 75 countries around the world.
Symplicity’s approval in Japan follows that of the Paradise ultrasound renal denervation system (Recor Medical) which received approval for manufacturing and marketing in Japan for the treatment of resistant hypertension last week.