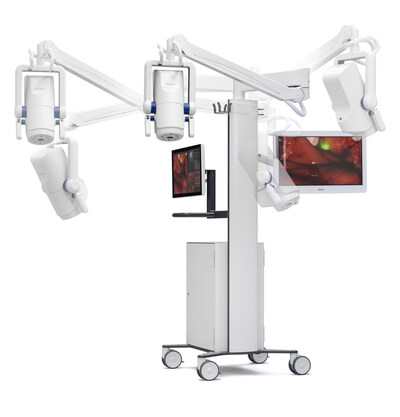
SurgVision, a company developing pioneering solutions for fluorescence-guided surgical and interventional oncology, part of the Bracco Group, has announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Explorer Air II for use with pafolacianine (Cytalux, On Target Laboratories) during intraoperative fluorescence imaging.
This is designed to achieve high-sensitivity and imaging fidelity, meeting the needs of oncological intraoperative fluorescence imaging. The system allows real-time imaging during surgery and its prototype has been tested by academic centres for a variety of indications.
“We are very excited about this important milestone. Our goal is to make the Explorer Air II available to surgeons, supporting them in their mission to fight cancer,” quoted Stefan Schorling, managing director and COO of SurgVision.
The Explorer Air II is also cleared in the USA and CE-marked in the EU for visual assessment of blood flow and tissue perfusion.
Currently, the identification of tumours during surgery or interventional endoscopy relies on visual inspection and palpation. Tumour tissue is sometimes difficult to distinguish from healthy tissue. As a consequence, surgical resection of the tumour is often incomplete. This has medical as well as financial implications, impairing patient treatment outcomes.