This advertorial is sponsored by Boston Scientific.
During a satellite symposium session dedicated to real cases and trial outcomes which was held at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress (14–18 September, Lisbon Portugal), experts presented compelling data from large registries and randomised controlled trials (RCTs) which suggest that the Eluvia drug-eluting stent (DES, Boston Scientific) should be preferred when treating the superficial femoral artery (SFA).

The Münster registry was led by Giovanni Torsello (St. Franziskus Hospital, Munster, Germany)—his son, Giovanni Federico Torsello (University Medical Center Göttingen, Göttingen, Germany) presented his research in his absence at the CIRSE annual congress.1 Torsello began by stating that the goal of real-world registries is to generate data which can be extrapolated to daily practice, considering all of the variables that are encountered day-to-day. To reflect this, the Münster registry assessed the performance of the Eluvia DES in long and complex femoropopliteal lesions, and assessed consecutive patients treated between 2016–2018 for stent primary patency.
The criteria for using the Eluvia DES, Torsello noted, was to treat recoil post-percutaneous transluminal angioplasty (PTA) or flow-limiting dissection post-PTA, labelling the registry the “bailout Eluvia DES study”. The likelihood of needing a bailout stent increases due to lesion length, chronic total occlusion (CTO) and calcification.
In the Münster all-comer cohort, 130 patients were enrolled totalling 137 lesions with a mean length of 194mm; 21% of included patients had a Rutherford classification of 5–6; 67% were identified as having a PACSS classification of 3 or 4; and 74% of patients had total occlusion of the SFA. Patients with Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC) A/B lesions and those with in-stent restenosis were excluded.
Key lessons from the Münster registry
Using a selection of cases which exemplify the Eluvia DES’s efficacy in a range of scenarios, Torsello pulled out key lessons from his experience treating long CTOs and displayed an exemplar case to depict this (Figure 1). He stated that a DES is necessary in these patients, as “if you don’t use a scaffold, you will not have mechanical stability—and if you use a scaffold without eluting an anti-restenotic drug, you will see in-stent restenosis”.
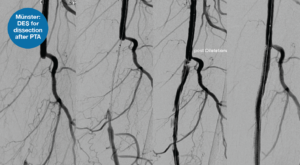
“What about calcium?” Torsello asked, moving on to show the “more difficult” calcification cases, which pose the added challenge of drug uptake and impaired expansion. He stated that, in these cases, often his team opt for upfront stenting of calcified occlusions paired with PTA to expand the DES. He added that short balloons should be used to exert more force in the segments which are heavily calcified and stenosed and promoted the use of a DES in this setting to benefit from lower rates of restenosis.
Taking what has been learnt and placing it within the context of the Münster registry, Torsello shared that the Eluvia DES showed 71% primary patency at two years, which increased to 78% if the entire lesion is covered by the DES, underlining its successful deployment in long lesions.
As published in the journal CardioVascular and Interventional Radiology (CVIR), the primary patency rate for the Eluvia DES was 65% at five years, with a clinically driven target lesion revascularisation (CD-TLR) and major amputation rate of 79% (Figure 2) and 96%, respectively.

Sustained high patency rates
“What we have seen is that you can successfully treat the SFA and downgrade patients’ disease with the Eluvia DES. What is important is a sustained high patency rate which we have achieved here, with results which are not unlike that of bypass but by endovascular means—it’s amazing to see,” Torsello commented.
Overall, he stated that the Eluvia DES offers a good bailout strategy in cases with a sub-optimal vessel preparation result such as dissection or recoil, or in complex lesions. In light of their long-term registry data, Torsello stated that he looks forward to the RCT data to come in this arena which he believes will bear out their outcomes and bolster Eluvia within the procedural standard.
Insights from SPORTS RCT support DES use in long, complex SFA lesions

“Truly long lesions have been underrepresented in randomised controlled trials [RCTs], so there were no definitive data on primary treatment strategy or decision making—the SPORTS trial was born out of limitation of current data on TASC C and D lesions for endovascular intervention”, were the opening statements of Gunnar Tepe (Klinikum Rosenheim, Rosenheim, Germany) who also presented during the satellite symposium session, sharing insights from the SPORTS RCT using the Eluvia drug-eluting stent (DES) in complex lesions.2
Initially, Tepe brought attention to the EMINENT and IMPERIAL (Boston Scientific) RCTs which each showed superior primary patency when using the Eluvia DES in the superficial femoral artery (SFA). The EMINENT trial—the largest RCT to compare drug-eluting to self-expanding bare metal stents— randomised 508 patients, and reported that Eluvia demonstrated superiority over bare metal stents, with a statistically significant primary patency of 85.4% versus 76.3% through to one year. The IMPERIAL trial, involving 465 randomised patients, compared the Eluvia DES to Zilver PTX (Cook Medical). At one year, the Eluvia device demonstrated a primary patency rate of 86.8%, compared to 77.5% for Zilver PTX. The longer sustained drug release from the Eluvia system was highlighted as a key differentiator.
SPORTS data updates DES patency
Tepe then situated the SPORTS RCT within the arena of other Eluvia trials, commenting that IMPERIAL and EMINENT were “early” explorations and concerned short lesions which minimised its broader application. Since then, these data have been updated with those gained from later trials, such as IMPERIAL Long and SPORTS, which have demonstrated the pertinency of the Eluvia DES in complex lesions while maintaining high patency rates.
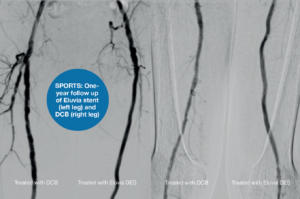
The SPORTS RCT was a prospective, randomised, multicentre trial which compared the angiographic and clinical outcomes of TASC C/D lesions in the SFA after treatment with the Eluvia DES and the SeQuent Please OTW (BBraun) drug-coated balloon (DCB), or a bare nitinol stent. Tepe—the trial’s principal investigator—and colleagues enrolled 224 patients with a mean lesion length of 228mm and a Rutherford classification of 2–4; 69% of patients were identified as having a PACSS classification of 3/4 and 76% had total occlusion of the SFA.
At one year, concerning angiographic stenosis, the data revealed superior results for the Eluvia DES (Figure 3) when compared to the bare nitinol stent. The SeQuent DCB was non-inferior to the bare nitinol stent. Of their secondary endpoints, Tepe noted that late lumen loss at 12 months “differed significantly” between treatment groups, however the least loss was reported in the Eluvia DES group.
Tepe situated the SPORTS RCT among others that have investigated the use of non-drug-eluting stents, showing that patency rates decrease when these are used in longer lesions. “With the Eluvia DES we have a really good patency even in those long, hard-to-treat lesions,” he continued.
“The SPORTS RCT provided new data on primary strategies in TASC C/D lesions and confirmed the Eluvia DES as superior to operator ‘best choice’ bare metal stenting for full lesion coverage,” Tepe stated. He described how the use of a DCB with or without bailout spot stenting as a strategy was non-inferior to full lesion bare metal stenting, however long bare metal stent restenosis remains “problematic”. This, Tepe stated, is due to the price of target lesion revascularisation (TLR) which can cost more than 22,000 euro per procedure.3
He concluded his talk by asserting that further analysis is underway to investigate the “astonishing” number of TLRs and failures in the DCB group, to create core-lab adjudicated “failure modes” for each treatment strategy.
Which gaps should future data fill?
Following Tepe’s talk, session moderator Marianne Brodmann (Medical University of Graz, Graz, Austria) asked the presenters what data is still missing concerning treatment of the SFA and what is needed now to guide daily practice and build a treatment algorithm. Tepe highlighted vessel preparation and the lack of data there, and noted that data to determine which device is superior in this setting would be beneficial, but conduction a study on this would be “very difficult”.
Sabine Steiner (Medical University Leipzig, Leipzig, Germany)—who also presented insights from the BEST SFA trial cases during the session—answered next, agreeing that the heterogeneity of SFA lesions makes conducting clinical trials in this space near impossible. “We need extremely good registry data with core-lab adjudication from angiogram. This is a prerequisite, because we know what investigators are reporting is in part ‘wishful thinking’,” Steiner said. She stated that intravascular ultrasound (IVUS) might assist in this area in the future, to “truly see what has an impact on our outcomes”.
In his response to Brodmann’s question, Torsello emphasised post-interventional medication and the lack of uniformity in its usage. He stated that, although studies such as VOYAGER have provided data on this, its takeaways “don’t apply to every patient”. Additionally, Torsello stated that research concerning the profunda femoris artery would be interesting, which is an area often overlooked in studies concerning the common femoral artery.
In conclusion, the Münster registry and SPORTS RCT presented at CIRSE provide compelling evidence for the sustained efficacy of the Eluvia DES in treating long, complex SFA lesions. The high primary patency rates displayed in these trials underscore the effectiveness of this endovascular approach. The Eluvia DES has demonstrated its value as a reliable bailout strategy in challenging cases, particularly where vessel preparation is suboptimal. Looking ahead, further research, including further RCTs and improved real-world registry data, will be crucial in addressing current challenges, such as restenosis in bare metal stents and high TLR rates in DCB treatments.
References
- Muenster: Stavroulakis, Konstantinos & Torsello, Giovanni & Bosiers, Michel & Argyriou, Angeliki& Tsilimparis, Nikolaos & Bisdas, Theodosios. (2021). 2-Year Outcomes of the Eluvia Drug-Eluting Stent for the Treatment of Complex Femoropopliteal Lesions. JACC: Cardiovascular Interventions. 14. 692-701.10.1016/j.jcin.2021.01.026.
- Tepe, G. SPORTS Trial: Drug Eluting Stent or Primary Bare Nitinol Stent Application versus Drug Coated Balloons in Long SFA Lesions. Presented at TCT 24 Oct 2023.
- Saratziset al. COST Analysis of Target Lesion Revascularisation in Patients With Femoropopliteal In-Stent Restenosis or Occlusion: The COSTLY-TLR Study. EurJ Vasc EndovascSurg. 2024 Feb 6:S1078-5884(24)00160-6. doi: 10.1016/j.ejvs.2024.02.001. Epub ahead of print. PMID: 38331163.