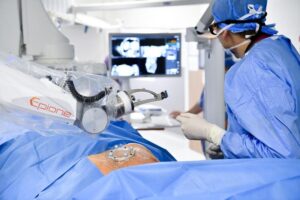
Quantum Surgical has announced that it has obtained CE mark approval for the expanded use of its Epione robotic platform in the treatment of bone tumours and metastases.
A recent press release made by the company states that the platform can also be used for bone consolidation. It assists physicians in performing percutaneous tumour ablations, where one or more needles are inserted through the skin to destroy the tumour. This minimally invasive treatment provides patients with an alternative to surgery. The release continues, describing that Epione allows physicians to treat inoperable tumours that are particularly difficult to reach, due to their size or their location, at an early stage.
Epione could, until now, be used to treat tumours in the abdomen (including the liver, kidneys, and pancreas) and the in the chest (lung). Over 1,000 patients have already been treated in Europe and the United States.
Based on a clinical study conducted in three French hospitals—Gustave Roussy in Villejuif, the Paoli-Calmettes Institute in Marseille and the Hospices Civils de Lyon in Lyon. The CE mark approval for bone tumours will expand this minimally invasive treatment to new patients in Europe.
“Given that a third cancer patients suffer from bone metastasis and weaknesses, which can be very painful, the entire Quantum Surgical team is delighted to obtain the new CE mark. New patients in Europe will be able to benefit from our innovative technology and will therefore experience a more comfortable healthcare pathway,” says Bertin Nahum, chief executive officer and co-founder of Quantum Surgical.
“The clinical study conducted at Gustave Roussy has demonstrated the relevance of Epione in the practice of bone percutaneous procedures. We will keep integrating robotics in these procedures which allow for better patient care,” explains Baptiste Bonnet, interventional radiologist at Gustave Roussy.
Laetitia Messner, chief clinical officer at Quantum Surgical adds: “Conducted by Bonnet, the clinical study has demonstrated the clinical performance and safety of Epione in the treatment of bone lesions and, more broadly, in the execution of percutaneous bone procedures, which until now had been performed manually. The expanded care offering provided by Epione will support both physicians and patients throughout the therapeutic pathway”.
Epione is currently available in approximately fifteen hospitals in Europe and in the USA. The device is CE marked for abdomen, chest and musculoskeletal structures indications, and US Food and Drug Administration (FDA) cleared for abdominal ablation indication.










