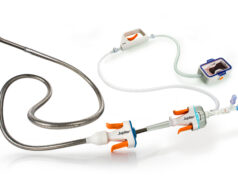
Preliminary in vitro data on Anaconda Biomed’s advanced thrombectomy system showed that the reperfusion rates with this novel system outperformed competing mechanical thrombectomy devices at first pass and after three passes. These results were presented by Tommy Andersson, (professor of Neurointervention, AZ Groeninge, Kortijk, Belgium, and senior consultant in Neurointervention, Department of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden) at the 39th ABC-WIN meeting in Val d’Isère, France.
The in vitro study compared the use of the advanced thrombectomy system in conjunction with a stent retriever (Solitaire, Medtronic) to a balloon-guide catheter with a stent retriever (BGC and Solitaire), and to an intermediate distal access catheter with a stent retriever (DAC and Solitaire).
The study results demonstrated that, as compared to the other device combinations, the advanced thrombectomy system achieved statistically significant improvement in revascularisation rates at both first and third pass. In fact, using the system, operators were able to achieve complete recanalisation [Thrombolysis in Cerebral Infarction (TICI) grade of 2b/3] in 100% of cases, with an average of 1.06 passes. Operators also found that the system’s design enabled complete local flow restriction and allowed for a safe exit.
“Mechanical thrombectomy techniques and devices have improved significantly over the years, but limitations remain. These range from difficulties with distal blood flow arrest and clot fragmentation to no or low reperfusion at first pass,” Andersson remarks. “In the in vitro model, with the advanced thrombectomy system, operators were able to overcome these challenges. Assuming these results can be replicated clinically, I believe this system has tremendous potential for fast, effective yet safe endovascular treatment of patients suffering from a major stroke.”
Anaconda’s advanced thrombectomy system consists of a delivery catheter, a unique, funnel-shaped aspiration catheter and a stent retriever. When deployed, the funnel self-expands and directly conforms to the artery diameter up to 5mm, locally arresting flow and allowing full thrombus extraction without fragmentation.
The company are currently planning future clinical studies for the advanced thrombectomy system.