Recor Medical has become the first company in the USA to have a device-based therapy approved for the treatment of hypertension, after it was announced that the US Food and Drug Administration (FDA) has approved the company’s Paradise ultrasound renal denervation (RDN) system this week.
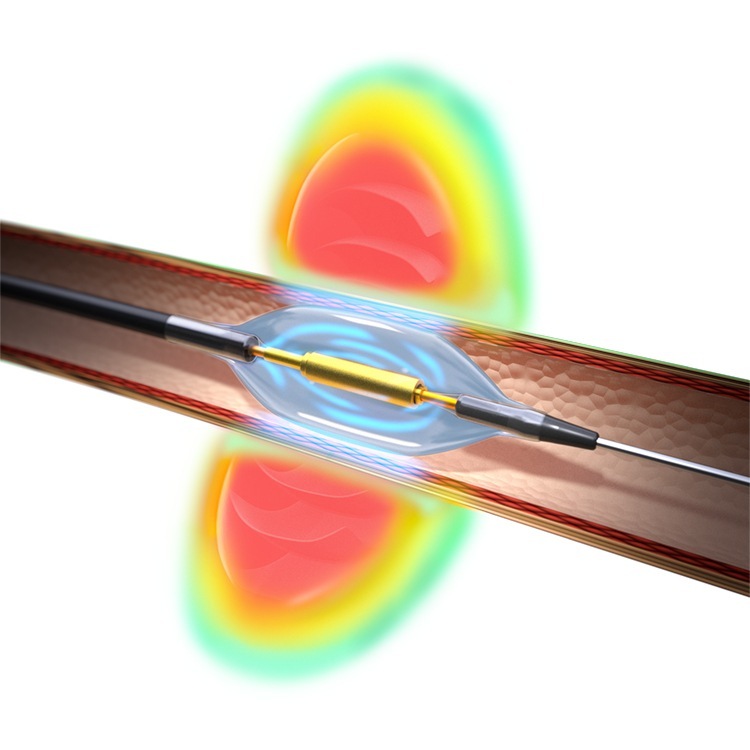
The Paradise system is intended as an adjunctive treatment option when lifestyle changes and medications have not adequately controlled a patient’s blood pressure. The Paradise catheter features the HydroCooling system, which circulates sterile water through the balloon catheter during the procedure to help protect the renal artery wall.
Approval of the Paradise system follows Recor’s positive FDA Advisory Committee Panel in August 2023. Earlier this year, results from Recor’s US pivotal study, the RADIANCE II randomised clinical trial, were published in the Journal of the American Medical Association (JAMA). In the study, the Paradise system met both the primary safety and effectiveness endpoints without any major adverse events.
“Despite the longstanding availability of dozens of affordable anti-hypertensive medications, blood pressure control rates in the USA are alarmingly low and falling. Given the significant blood pressure reductions seen in the ultrasound renal denervation trials, the Paradise ultrasound renal denervation system offers a much-needed advancement in our currently available options to control hypertension,” said site principal investigator Naomi Fisher (Brigham and Women’s Hospital, Boston, USA). “Ultrasound renal denervation has proven efficacy in patients with truly resistant hypertension, a population for whom medication therapy often fails. It is also effective in patients with mild to moderate hypertension who cannot tolerate enough medication to control their blood pressure.”
The Paradise system previously received CE mark and has been successfully introduced in Europe and is an investigational device in Japan.
“Recor is leading the way in bringing an innovative solution to clinicians and their patients struggling to control blood pressure. This FDA approval is the culmination of years of technical research and rigorous clinical studies,” said Lara Barghout, President and CEO of Recor Medical. “We are grateful to the patients who participated in the studies and to the clinical trial investigator teams whose diligence and dedication made FDA approval possible. We look forward to making this technology available to physicians and their patients nationwide.”
“Approval of the Paradise ultrasound renal denervation system marks an important milestone for the company and provides a new adjunctive treatment option for hypertension which remains inadequately controlled despite conventional therapies,” said Noriko Tojo, president and representative director of Otsuka Medical Devices and executive director of Otsuka Holdings Co, Ltd, the parent company of Recor Medical. “We are excited for patients and their healthcare professionals to have access to this technology to assist in managing hypertension and improving outcomes.”