Thomas Zeller, University Heart Center, Freiburg-Bad Krozingen, Germany, presented results of the first clinical experience with the BioMimics 3D stents at the VIVA conference in Las Vegas, USA.
The data have shown that the three-dimensional stent demonstrated an excellent safety profile and promising clinical performance at both six and 12 months in the treatment of patients with femoropopliteal lesions.
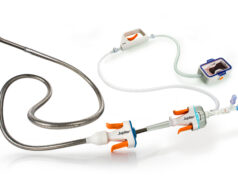
“This is a completely new stent design regarding nitinol stents. The unique feature of this device is a centreline helical curvature that mimics the natural helical geometry of the human vascular system. The overall goal of this design is to reduce vessel trauma and improve fracture resistance,” Zeller said.
The helical stent design converts laminar flow into a swirling flow pattern inside the stent. “This increases shear stress and results in a significant reduction in mean intimal thickness as shown in thirty-day histological results in porcine carotid arteries,” Zeller stated.
The MIMICS study is a prospective, randomised controlled trial, conducted at eight German investigational centres, comparing the safety and efficacy of the BioMimics 3D (Veryan) stent with a straight nitinol stent (primarily the Bard LifeStent) in patients undergoing femoropopliteal artery intervention. Zeller is the principal investigator in the trial.
In the study, 50 patients received the BioMimics 3D stent and 26 received the control stent. At six months, all patients in both treatment groups were free from clinically-driven target lesion revascularisation, and there were no deaths or amputations. Of the 36 patients treated with BioMimics 3D stents who had reached the 12-month follow-up time point, 33 (91.7%) remained free from clinically-driven target lesion revascularisation, compared with 18 of the 21 (85.7%) from the control group who reached the same follow-up time point. The independent core lab, Zeller said, has not detected any stent fractures to date in either treatment group.
“The unique BioMimics 3D stent architecture could be applied to all current slotted tube nitinol stents, including drug-eluting stents, potentially improving their long-term stent integrity,” said Zeller. “The final 12-month results will show if the induction of a swirling flow by the 3D architecture, and therefore an increase in wall shear stress, affects patency. Ongoing two- and three-year plain X-ray follow-up will show if there is a difference in longer-term stent integrity over time.”
Veryan has submitted a CE mark application for the BioMimics 3D stent.