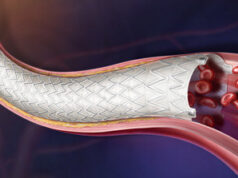
In a new post-hoc analysis of the Lutonix (BD) arteriovenous (AV) global registry using investigational device exemption (IDE) criteria, Dimitrios Karnabatidis (Patras University Hospital, Patras, Greece) reports the “highest” six-month target lesion primary patency (TLPP) to date when evaluated through IDE criteria, presented at the Endo Vascular Access (EVA) meeting (14-15 June, Patras, Greece).
As part of a post-approval study mandated by the US Food and Drug Administration (FDA), the Lutonix AV global registry assessed the safety and clinical benefit of the Lutonix drug-coated balloon (DCB) catheter for the treatment of dysfunctional AV fistulae (AVF) and grafts (AVG) located in the arm. The real-world, multicentre, prospective study enrolled 320 patients from 12 countries across Europe and Asia—the largest DCB arm to date— Karnabatidis told EVA delegates.
At six months, the primary safety endpoint defined as freedom from serious adverse events was reported as 95.5%, while TLPP was 73.9% and access circuit primary patency (ACPP) was 71%. TLPP for stenosis of AVFs was 78.1%.
Karnabatidis then went on to question how key IDE exclusion criteria would affect these results, detailing which variables would affect the patient population. These variables include:
- Lesions of >80mm
- Lesions outside the range of 4–12mm
- >30% residual stenosis
- Previous graft
- In-stent restenosis
- Central veins as target lesions
Karnabatidis and his team also identified thrombosis at index as a potential exclusory variable, comparing this to the IN.PACT IDE trial which also excluded patients with current or previous thrombosis events. Other key exclusions include the presence of a stent in the target or secondary lesion, or if more than two secondary lesions are in circuit, however Karnabatidis specified that if a single patent stent is in circuit this was allowed.
Using IDE exclusion criteria to better understand the Lutonix registry results, Karnabatidis stated that the primary safety endpoint of freedom from access circuit-related serious adverse events was 94.9% at 30 days. Concerning the registry’s efficacy endpoint, TLPP was 85.1% at 180 days—the “highest” reported when compared with the original dataset. Karnabatidis concluded that often DCB trials are impacted by procedural variables such as inflation time, variations in dose when delivering drugs and excipients and patient population differences. For these reasons, it is challenging to compare DCB trials, he said, underscoring the importance of future randomised controlled trials in this area.