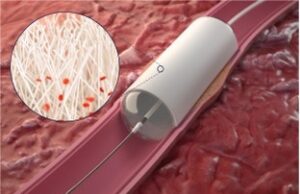
Cryoablation gains ground as effective alternative treatment for T1a renal cell carcinoma in EuRECA...
Interim analysis from the prospective multinational European Registry for Renal Cryoablation (EuRECA)—aimed to assess the oncological outcomes and safety profile of percutaneous cryoablation for...
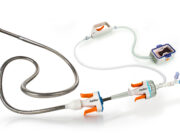
Jupiter Endovascular completes enrolment in SPIRARE II pivotal trial
Jupiter Endovascular today announced completion of patient enrolment in the SPIRARE II pivotal clinical trial evaluating the Vertex pulmonary embolectomy system in patients with...
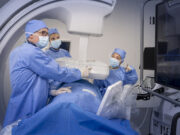
GE HealthCare’s Allia Moveo gains FDA and CE mark approvals
GE HealthCare has announced that its Allia Moveo image-guidance platform has received US Food and Drug Administration (FDA) 510(k) clearance and CE mark.
The latest...
Boston Scientific appoints Isabel Newton as chief medical officer of its interventional oncology and...
Boston Scientific has announced that Isabel Newton has been named chief medical officer of the company’s interventional oncology (IO) and embolization business.
The company...