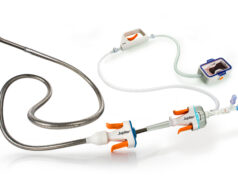
Jupiter Endovascular has announced that the US Food and Drug Administration (FDA) has approved its investigational device exemption (IDE) application for the SPIRARE II US pivotal study. The pivotal trial will study the Vertex pulmonary embolectomy system, which incorporates Jupiter’s Endoportal Control platform technology into endovascular procedures intended to treat acute pulmonary embolism (PE).
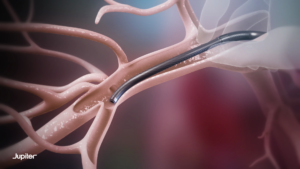
Endoportal Control is designed to bring these benefits to a variety of catheter interventions, with the goal of enabling interventionalists to deliver treatment to anatomical sites that they cannot safely or easily reach with a conventional endovascular approach. The endoportal device is delivered in a relaxed state over a guidewire to the target location in the vasculature, pressurised with saline to fix it in a stable position for therapeutic delivery, then relaxed again to navigate to another target location or for removal.
SPIRARE II is a prospective, single-arm, multicentre pivotal trial that will enrol up to 145 patients with acute, intermediate-risk PE treated with the Vertex PE system at up to 25 US sites, a recent press release states. Trial endpoints will characterise the procedural and clinical benefits of PE treatment with Endoportal Control using the Vertex system, across measures of safety, right heart function, and clinical improvement from the time of the procedure to 30 days post-procedure. SPIRARE II is part of Jupiter Endovascular’s SPIRARE clinical program, which also includes the SPIRARE I clinical study evaluating the Vertex system at up to two sites in Europe.
“One of the remaining challenges in treating PE is the ability to safely navigate through the right heart and into multiple areas of the pulmonary vasculature. The act of placing stiff interventional devices across the right heart may add stress to a strained right ventricle, and today’s catheters can become difficult to purposefully control beyond the main pulmonary arteries,” said Catalin Toma, director of Interventional Cardiology for the Heart and Vascular Institute at the University of Pittsburgh Medical Center (UPMC) and national co-principal investigator for the SPIRARE II trial. “These risks pose a barrier to the widespread adoption of large-bore catheter therapies for PE, creating the need for a procedural system that can safely access the pulmonary arteries with increased control.”
“In open surgery, we have the benefit of being able to directly visualise the anatomy and the instruments with which we are working. This provides surgeons with an excellent level of control and precision,” said Joshua Goldberg, surgical director of the Structural Heart Surgery Program at Weill Cornell Medical Center in New York and national co-principal investigator of SPIRARE II. “A percutaneous embolectomy system that can provide interventionalists with the control and stability we enjoy in surgery holds the potential to dramatically improve procedural safety, efficacy, and efficiency.”
“We are excited about FDA approval of the first pivotal study of a system leveraging our Endoportal Control platform technology. In the more than 25 animal studies conducted to-date with the technology, it has demonstrated safe and easy navigation through the heart and vasculature, and effective delivery of test interventions,” said Carl J St Bernard, Jupiter Endovascular CEO. “Endoportal Control has the potential to meaningfully improve many endovascular procedures while enabling entirely new therapies. Our Vertex system for pulmonary embolectomy is the first of what we intend to be a portfolio of our own interventional procedure systems incorporating Endoportal Control to treat a variety of cardiovascular conditions that affect millions of patients worldwide.”