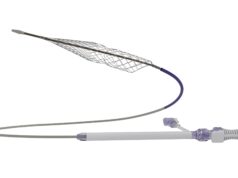
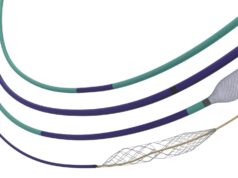
Inari Medical has announced that it received national reimbursement approval from the Japanese Ministry of Health, Labor and Welfare (MHLW) for its ClotTriever thrombectomy system for deep vein thrombosis (DVT).
A recent press release states that, due to ClotTriever’s mechanism of action for wall-to-wall thrombus removal in patients suffering from DVT, the MHLW have created a new functional category that is separate from other catheter-based therapies. This new functional category comes with a reimbursement premium based on clinical data showing the system’s efficacy in removing various types of thrombi.
To facilitate commercialisation of the ClotTriever system in Japan, Inari has entered into a distribution agreement with Medikit, a vascular medical device manufacturer serving Japan, the USA and over 30 other countries worldwide. With this new partnership, Inari plans to accelerate initiation of its 100-patient post market surveillance study.
“MHLW’s approval of reimbursement for ClotTriever under a newly designated functional category marks a transformative milestone for Inari in Japan,” said Drew Hykes, chief executive officer of Inari. “This decision underscores the value of ClotTriever in addressing unmet clinical needs, and we are thrilled to collaborate with Medikit to bring this innovative solution to Japanese DVT patients, improving lives and advancing care in the near future. Over time, we look forward to bringing our broader portfolio of purpose-built tools to the Japanese market.”
The ClotTriever system is 510k-cleared by US Food and Drug Administration and CE-marked for treatment of DVT. More than 75,000 procedures have been conducted with ClotTriever globally. Recently, two-year outcomes were reported from the 500-patient ClotTriever CLOUT registry showing a strong safety profile, significant clot removal, and low rates of post-thrombotic syndrome.