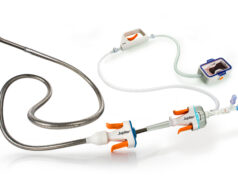
The US Food and Drug Administration (FDA) has issued a Class I recall—the most serious type—for Inari Medical‘s ClotTriever XL, 30mm device due to reports of patient injury, and death from device entrapment and pulmonary emboli (PE).
The device remains on the market with updated instructions for use (IFU), with all devices and lot numbers with labelled dates prior to 1 August affected. The FDA recall was categorised as a “correction,” addressing a problem with a medical device in the place where it is used or sold rather than removing it from the market.
There have been four reported injuries and six reports of death, the FDA noted. The serious adverse events occurred in patients who had the ClotTriever catheter inserted through the jugular vein; thrombus that is fibrotic, organised and/or adherent; clot formed by tumour cells; and extremely large clot that cannot be removed in pieces.
On 19 July 2024, Inari sent all affected customers an Urgent Medical Device Labeling Correction letter covering IFU updates, recommending that they view the letter and updates, then share it with any relevant personnel and/or device users; share this information with any organisation where the ClotTriever XL catheter may have been transferred; and complete the Customer Acknowledgement Reply Form as soon as possible.
The FDA recommended that, based on its review of the reported adverse events, users consider the device to be contraindicated for the removal of fibrous, firmly adherent or calcified material, and be aware that the use of a clot capture device—for example an embolic protection device—”has not yet been demonstrated to be effective in the venous vasculature.”
The FDA noted in the recall that US customers with questions about the recall should contact their local Inari sales representative, Inari Customer Care at 877-923-4747, or its quality department at [email protected].