The US Food and Drug Administration (FDA) has granted marketing authorisation for IceCure Medical’s ProSense, a minimally invasive cryoablation treatment for patients with early stage low-risk breast cancer when combined with adjuvant endocrine therapy for women aged 70 and over.
“We are excited to add a minimally invasive choice around breast cancer treatments and to offer patients an effective, outpatient procedure,” said Eyal Shamir, chief executive officer, IceCure. “With the ProSense cryoablation system, we are giving women with low-risk, early-stage breast cancer the choice to freeze their cancer, not their lives, through an effective treatment that minimizes recovery time, and minimal cosmetic changes to the breast.”
In a recent press release the company has stated that ProSense is the first and only medical device to be granted FDA marketing authorisation for the local treatment of breast cancer.
ProSense is authorised by the FDA for the local treatment of breast cancer in patients ≥70 years of age with biologically low-risk tumours ≤1.5cm in size and treated with adjuvant endocrine therapy. Biologically low-risk breast cancer is defined as unifocal tumor, size ≤1.5cm, ER+, PR+, HER2-, Ki-67<15% and/or genomic testing indicative of low-risk breast cancer, infiltrating ductal carcinoma (excluding lobular carcinoma, extensive intraductal component, or evidence of lymphovascular invasion), and clinically negative lymph node (N0). The authorised indication includes patients that are not suitable for surgery for breast cancer treatment.
In granting marketing authorisation, the FDA requested that IceCure conduct a post-market surveillance study with the aim of producing additional data in this indication. The post-market study is expected to include approximately 400 patients at 30 sites.
“You don’t need any kind of cosmetic follow-up, you don’t have a scar, and you don’t have the feeling of having lost part of your breast, because it’s all still there,” said breast cancer patient and ICE3 trial participant, Pam Dixon, when describing her experience with the ProSense cryoablation procedure. “There was no pain. It was one of the easiest things I’ve ever done. I don’t remember any limitations on my activity.”
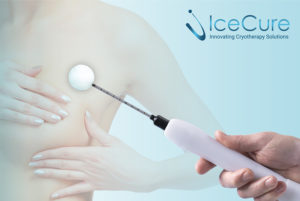
During the ProSense cryoablation procedure, a doctor injects local anaesthesia and uses ultrasound imaging to guide a small cryoprobe, a thin hollow needle, into the breast tumour. Once the cryoprobe is placed, liquid nitrogen creates extremely cold temperatures (-170C°) which destroys the breast tumour by creating an ice ball around the targeted tissue. Key advantages of ProSense cryoablation procedure include:
The procedure is monitored in real-time by ultrasound to ensure the ice ball is growing sufficiently around the tumour, and to avoid damage to the skin or muscle. The doctor may use hydro-dissection to protect the skin or muscle during a procedure depending on the location of the tumour. The tissue destroyed by the ice ball is naturally reabsorbed by the body over time and adjacent tissue is left unharmed.
The FDA’s marketing authorisation was based on an abundance of data including IceCure’s ICE3 trial which was published in the Annals of Surgical Oncology. With 194 patients, ICE3 is the largest multicentre clinical trial ever completed for liquid-nitrogen (LN2) based cryoablation for patients aged ≥60 with low-risk, early-stage breast cancer without surgically removing the breast tumour. Only 3.1% of patients with hormone receptor-positive and HER2-breast cancer treated locally with cryoablation and endocrine therapy experienced local recurrence of breast cancer within five years after treatment, based on the study results.
The majority of the cryoablation procedure-related adverse events besides breast cancer recurrence were oedema (swelling), bruising, haematoma (bleeding into tissues), skin burn, and postoperative pain. These were mild in severity and all these events resolved without any permanent effect.
ICE3 study lead author, Richard Fine, of the West Cancer Center & Research Institute in Germantown, USA and past president of the American Society of Breast Surgeons emphasises that: “The ICE3 study has proven that cryoablation with ProSense is a safe, minimally invasive ablative procedure with results similar to that of lumpectomy patients who took endocrine therapy, and has the benefit of being an office-based, non-surgical treatment. Further data coming out of the post-market study should continue to support that cryoablation with ProSense is a successful option in the de-escalation of breast cancer care in appropriately selected patients.”