Hansen has announced that it will present its Magellan Robotic System and its new 6F Robotic Catheter at the Society of Interventional Radiology’s (SIR) Annual Scientific Meeting (23–27 March, San Diego, USA). The company will be conducting product demonstrations and exhibiting the system at Booth 800.
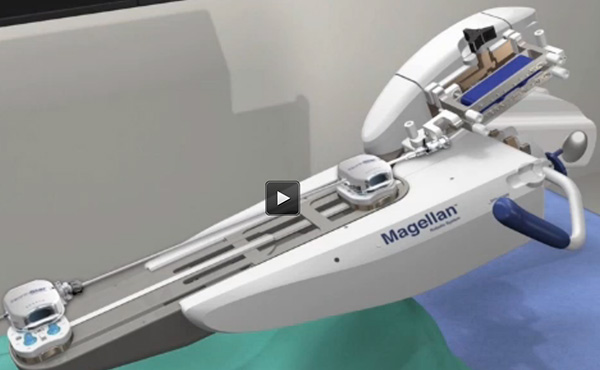
The Magellan Robotic System is intended to be used to facilitate navigation in the peripheral vasculature and subsequently provide a conduit for manual placement of therapeutic devices. The Magellan Robotic System is designed to deliver improved predictability, control and catheter stability to endovascular procedures. The system employs an open architecture designed to allow for the subsequent use of many therapeutic devices on the market today and is designed to potentially reduce physician radiation exposure and fatigue by allowing the physician to navigate procedures while seated comfortably at a remote workstation away from the radiation field and without wearing heavy lead as required in conventional endovascular procedures.
Hansen Medical will also be sponsoring a dinner symposium during the meeting titled “Clinical Experiences with the Magellan Robotic System”. Barry Katzen, and Constantino Peña, both from Baptist Cardiac and Vascular Institute in Miami, USA will share their experiences with the Magellan Robotic System in a wide variety of endovascular procedures. They will also discuss the new Magellan 6F Robotic Catheter and its potential to expand the role of intravascular robotics to include peripheral vascular procedures in smaller vessels.
Chris Lowe, Interim CEO, Hansen Medical said: “With the recent FDA 510(k) clearance for our Magellan 6F Catheter, we will be expanding the clinical applications for intravascular robotics to include procedures in smaller diameter vessels, many of which are performed by interventional radiologists.”
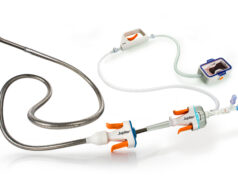
The Magellan 9F Robotic Catheter is for independent, robotic control of the distal tip of two telescoping catheters (an outer guide and an inner leader catheter), as well as robotic manipulation of standard guidewires. The 6F Robotic Catheter allows for independent robotic control of two separate bend sites on a single catheter, as well as robotic manipulation of standard guidewires. This smaller catheter design may be preferred by certain physicians who prefer a smaller diameter vessel access site, or in procedures in smaller vessels.